The Convergence of Alpha-Synuclein, Mitochondrial, and Lysosomal Pathways in Vulnerability of Midbrain Dopaminergic Neurons in Parkinson's Disease
- PMID: 33381501
- PMCID: PMC7767856
- DOI: 10.3389/fcell.2020.580634
The Convergence of Alpha-Synuclein, Mitochondrial, and Lysosomal Pathways in Vulnerability of Midbrain Dopaminergic Neurons in Parkinson's Disease
Abstract
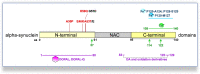
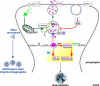
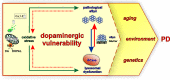
Parkinson's disease (PD) is the second most common neurodegenerative disease, characterized by progressive bradykinesia, rigidity, resting tremor, and gait impairment, as well as a spectrum of non-motor symptoms including autonomic and cognitive dysfunction. The cardinal motor symptoms of PD stem from the loss of substantia nigra (SN) dopaminergic (DAergic) neurons, and it remains unclear why SN DAergic neurons are preferentially lost in PD. However, recent identification of several genetic PD forms suggests that mitochondrial and lysosomal dysfunctions play important roles in the degeneration of midbrain dopamine (DA) neurons. In this review, we discuss the interplay of cell-autonomous mechanisms linked to DAergic neuron vulnerability and alpha-synuclein homeostasis. Emerging studies highlight a deleterious feedback cycle, with oxidative stress, altered DA metabolism, dysfunctional lysosomes, and pathological alpha-synuclein species representing key events in the pathogenesis of PD. We also discuss the interactions of alpha-synuclein with toxic DA metabolites, as well as the biochemical links between intracellular iron, calcium, and alpha-synuclein accumulation. We suggest that targeting multiple pathways, rather than individual processes, will be important for developing disease-modifying therapies. In this context, we focus on current translational efforts specifically targeting lysosomal function, as well as oxidative stress via calcium and iron modulation. These efforts could have therapeutic benefits for the broader population of sporadic PD and related synucleinopathies.
Keywords: Parkinson’s disease; alpha-synuclein; calcium; dopamine; iron; mitochondria; synapse.
Copyright © 2020 Minakaki, Krainc and Burbulla.
Conflict of interest statement
DK was the Founder and Scientific Advisory Board Chair of Lysosomal Therapeutics Inc., and Vanqua Bio. DK serves on the scientific advisory boards of The Silverstein Foundation, Intellia Therapeutics, and Prevail Therapeutics and is a Venture Partner at OrbiMed. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aflaki E., Borger D. K., Moaven N., Stubblefield B. K., Rogers S. A., Patnaik S., et al. (2016). A New Glucocerebrosidase Chaperone Reduces alpha-Synuclein and Glycolipid Levels in iPSC-Derived Dopaminergic Neurons from Patients with Gaucher Disease and Parkinsonism. J. Neurosci. 36 7441–7452. 10.1523/JNEUROSCI.0636-16.2016 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

