Chemical-Induced Cleft Palate Is Caused and Rescued by Pharmacological Modulation of the Canonical Wnt Signaling Pathway in a Zebrafish Model
- PMID: 33381503
- PMCID: PMC7767894
- DOI: 10.3389/fcell.2020.592967
Chemical-Induced Cleft Palate Is Caused and Rescued by Pharmacological Modulation of the Canonical Wnt Signaling Pathway in a Zebrafish Model
Abstract
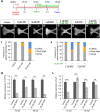
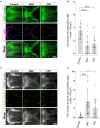
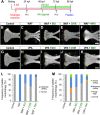
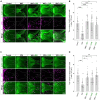
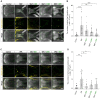
Cleft palate is one of the most frequent birth defects worldwide. It causes severe problems regarding eating and speaking and requires long-term treatment. Effective prenatal treatment would contribute to reducing the risk of cleft palate. The canonical Wnt signaling pathway is critically involved in palatogenesis, and genetic or chemical disturbance of this signaling pathway leads to cleft palate. Presently, preventative treatment for cleft palate during prenatal development has limited efficacy, but we expect that zebrafish will provide a useful high-throughput chemical screening model for effective prevention. To achieve this, the zebrafish model should recapitulate cleft palate development and its rescue by chemical modulation of the Wnt pathway. Here, we provide proof of concept for a zebrafish chemical screening model. Zebrafish embryos were treated with 12 chemical reagents known to induce cleft palate in mammals, and all 12 chemicals induced cleft palate characterized by decreased proliferation and increased apoptosis of palatal cells. The cleft phenotype was enhanced by combinatorial treatment with Wnt inhibitor and teratogens. Furthermore, the expression of tcf7 and lef1 as a readout of the pathway was decreased. Conversely, cleft palate was prevented by Wnt agonist and the cellular defects were also prevented. In conclusion, we provide evidence that chemical-induced cleft palate is caused by inhibition of the canonical Wnt pathway. Our results indicate that this zebrafish model is promising for chemical screening for prevention of cleft palate as well as modulation of the Wnt pathway as a therapeutic target.
Keywords: canonical Wnt signaling pathway; cleft palate; environmental factors; teratogen; zebrafish.
Copyright © 2020 Narumi, Liu, Ikeda, Morita and Tasaki.
Conflict of interest statement
All authors are employed by the company Kao Corporation.
Figures

Similar articles
-
Modulating Wnt Signaling Rescues Palate Morphogenesis in Pax9 Mutant Mice.J Dent Res. 2017 Oct;96(11):1273-1281. doi: 10.1177/0022034517719865. Epub 2017 Jul 10. J Dent Res. 2017. PMID: 28692808 Free PMC article.
-
Small-molecule Wnt agonists correct cleft palates in Pax9 mutant mice in utero.Development. 2017 Oct 15;144(20):3819-3828. doi: 10.1242/dev.157750. Epub 2017 Sep 11. Development. 2017. PMID: 28893947 Free PMC article.
-
Gpr177-mediated Wnt Signaling Is Required for Secondary Palate Development.J Dent Res. 2015 Jul;94(7):961-7. doi: 10.1177/0022034515583532. Epub 2015 Apr 28. J Dent Res. 2015. PMID: 25922332
-
Cellular and molecular mechanisms in the development of a cleft lip and/or cleft palate; insights from zebrafish (Danio rerio).Anat Rec (Hoboken). 2021 Aug;304(8):1650-1660. doi: 10.1002/ar.24547. Epub 2020 Nov 2. Anat Rec (Hoboken). 2021. PMID: 33099891 Review.
-
Wnt signaling in lip and palate development.Front Oral Biol. 2012;16:81-90. doi: 10.1159/000337619. Epub 2012 Jun 25. Front Oral Biol. 2012. PMID: 22759672 Review.
Cited by
-
Bone morphogenetic protein signaling pathway- Ethanol interactions disrupt palate formation independent of gata3.Reprod Toxicol. 2025 Jan;131:108754. doi: 10.1016/j.reprotox.2024.108754. Epub 2024 Nov 23. Reprod Toxicol. 2025. PMID: 39586481
-
Pharmacological Inhibition of the Spliceosome SF3b Complex by Pladienolide-B Elicits Craniofacial Developmental Defects in Mouse and Zebrafish.Birth Defects Res. 2024 Nov;116(11):e2404. doi: 10.1002/bdr2.2404. Birth Defects Res. 2024. PMID: 39494782 Free PMC article.
-
Valproic acid-induced teratogenicity is driven by senescence and prevented by Rapamycin in human spinal cord and animal models.Mol Psychiatry. 2025 Mar;30(3):986-998. doi: 10.1038/s41380-024-02732-0. Epub 2024 Sep 3. Mol Psychiatry. 2025. PMID: 39227432 Free PMC article.
-
Astragalus polysaccharides and astragaloside IV alleviate inflammation in bovine mammary epithelial cells by regulating Wnt/β-catenin signaling pathway.PLoS One. 2022 Jul 25;17(7):e0271598. doi: 10.1371/journal.pone.0271598. eCollection 2022. PLoS One. 2022. PMID: 35877777 Free PMC article.
-
Expression analysis of genes including Zfhx4 in mice and zebrafish reveals a temporospatial conserved molecular basis underlying craniofacial development.Dev Dyn. 2025 Mar;254(3):257-271. doi: 10.1002/dvdy.740. Epub 2024 Sep 25. Dev Dyn. 2025. PMID: 39320016 Free PMC article.
References
-
- Brent R. L. (2004). Environmental causes of human congenital malformations: the pediatrician’s role in dealing with these complex clinical problems caused by a multiplicity of environmental and genetic factors. Pediatrics 113 957–968. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

