DIPPER, a spatiotemporal proteomics atlas of human intervertebral discs for exploring ageing and degeneration dynamics
- PMID: 33382035
- PMCID: PMC7857729
- DOI: 10.7554/eLife.64940
DIPPER, a spatiotemporal proteomics atlas of human intervertebral discs for exploring ageing and degeneration dynamics
Abstract
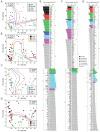
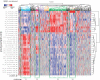
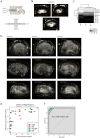
The spatiotemporal proteome of the intervertebral disc (IVD) underpins its integrity and function. We present DIPPER, a deep and comprehensive IVD proteomic resource comprising 94 genome-wide profiles from 17 individuals. To begin with, protein modules defining key directional trends spanning the lateral and anteroposterior axes were derived from high-resolution spatial proteomes of intact young cadaveric lumbar IVDs. They revealed novel region-specific profiles of regulatory activities and displayed potential paths of deconstruction in the level- and location-matched aged cadaveric discs. Machine learning methods predicted a 'hydration matrisome' that connects extracellular matrix with MRI intensity. Importantly, the static proteome used as point-references can be integrated with dynamic proteome (SILAC/degradome) and transcriptome data from multiple clinical samples, enhancing robustness and clinical relevance. The data, findings, and methodology, available on a web interface (http://www.sbms.hku.hk/dclab/DIPPER/), will be valuable references in the field of IVD biology and proteomic analytics.
Keywords: SILAC; ageing; computational biology; degradome; human; intervertebral disc; nucleus pulposus; proteomics; systems biology.
Plain language summary
The backbone of vertebrate animals consists of a series of bones called vertebrae that are joined together by disc-like structures that allow the back to move and distribute forces to protect it during daily activities. It is common for these intervertebral discs to degenerate with age, resulting in back pain and severely reducing quality of life. The mechanical features of intervertebral discs are the result of their proteins. These include extracellular matrix proteins, which form the external scaffolding that binds cells together in a tissue, and signaling proteins, which allow cells to communicate. However, how the levels of different proteins in each region of the disc vary with time has not been fully examined. To establish how protein composition changes with age, Tam, Chen et al. quantified the protein levels and gene activity (which leads to protein production) of intervertebral discs from young and old deceased individuals. They found that the position of different mixtures of proteins in the intervertebral disc changes with age, and that young people have high levels of extracellular matrix proteins and signaling proteins. Levels of these proteins decreased as people got older, as did the amount of proteins produced. To determine which region of the intervertebral disc different proteins were in, Tam, Chen et al. also performed magnetic resonance imaging (MRI) of the samples to correlate image intensity (which represents water content) with the corresponding protein signature. The data obtained provides a high-quality map of how the location of different proteins changes with age, and is available online under the name DIPPER. This database is an informative resource for research into skeletal biology, and it will likely advance the understanding of intervertebral disc degeneration in humans and animals, potentially leading to the development of new treatment strategies for this condition.
© 2020, Tam et al.
Conflict of interest statement
VT, PC, AY, NS, MK, RS, WC, CO, LH, PS, DC No competing interests declared, TK Theo Klein is affiliated with Triskelion BV. The author has no financial interests to declare, KC Senior editor, eLife
Figures



















Similar articles
-
Matrisome Profiling During Intervertebral Disc Development And Ageing.Sci Rep. 2017 Sep 14;7(1):11629. doi: 10.1038/s41598-017-11960-0. Sci Rep. 2017. PMID: 28912585 Free PMC article.
-
Inflammaging determines health and disease in lumbar discs-evidence from differing proteomic signatures of healthy, aging, and degenerating discs.Spine J. 2020 Jan;20(1):48-59. doi: 10.1016/j.spinee.2019.04.023. Epub 2019 May 22. Spine J. 2020. PMID: 31125691
-
Fibrotic-like changes in degenerate human intervertebral discs revealed by quantitative proteomic analysis.Osteoarthritis Cartilage. 2016 Mar;24(3):503-13. doi: 10.1016/j.joca.2015.09.020. Epub 2015 Oct 20. Osteoarthritis Cartilage. 2016. PMID: 26463451
-
Defects in intervertebral disc and spine during development, degeneration, and pain: New research directions for disc regeneration and therapy.Wiley Interdiscip Rev Dev Biol. 2019 Jul;8(4):e343. doi: 10.1002/wdev.343. Epub 2019 Apr 11. Wiley Interdiscip Rev Dev Biol. 2019. PMID: 30977275 Free PMC article. Review.
-
Role of the Wnt pathway in the formation, development, and degeneration of intervertebral discs.Pathol Res Pract. 2021 Apr;220:153366. doi: 10.1016/j.prp.2021.153366. Epub 2021 Feb 8. Pathol Res Pract. 2021. PMID: 33647863 Review.
Cited by
-
The Cellular Composition of Bovine Coccygeal Intervertebral Discs: A Comprehensive Single-Cell RNAseq Analysis.Int J Mol Sci. 2021 May 6;22(9):4917. doi: 10.3390/ijms22094917. Int J Mol Sci. 2021. PMID: 34066404 Free PMC article.
-
Biglycan fragment modulates TGF-β activity in intervertebral disc via an eIF6-coupled intracellular path.Sci Adv. 2025 Feb 14;11(7):eadq8545. doi: 10.1126/sciadv.adq8545. Epub 2025 Feb 14. Sci Adv. 2025. PMID: 39951526 Free PMC article.
-
Peptide Location Fingerprinting Reveals Tissue Region-Specific Differences in Protein Structures in an Ageing Human Organ.Int J Mol Sci. 2021 Sep 27;22(19):10408. doi: 10.3390/ijms221910408. Int J Mol Sci. 2021. PMID: 34638745 Free PMC article.
-
Experimental validation and comprehensive analysis of m6A methylation regulators in intervertebral disc degeneration subpopulation classification.Sci Rep. 2024 Apr 10;14(1):8417. doi: 10.1038/s41598-024-58888-w. Sci Rep. 2024. PMID: 38600232 Free PMC article.
-
Current cutting-edge omics techniques on musculoskeletal tissues and diseases.Bone Res. 2025 Jun 9;13(1):59. doi: 10.1038/s41413-025-00442-z. Bone Res. 2025. PMID: 40484858 Free PMC article. Review.
References
-
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. - PubMed
-
- Bizet AA, Liu K, Tran-Khanh N, Saksena A, Vorstenbosch J, Finnson KW, Buschmann MD, Philip A. The TGF-β co-receptor, CD109, promotes internalization and degradation of TGF-β receptors. Biochimica Et Biophysica Acta (BBA) - Molecular Cell Research. 2011;1813:742–753. doi: 10.1016/j.bbamcr.2011.01.028. - DOI - PubMed
-
- Braschi B, Denny P, Gray K, Jones T, Seal R, Tweedie S, Yates B, Bruford E. Genenames.org: the HGNC and VGNC resources in 2019. Nucleic Acids Research. 2019;47:D786–D792. doi: 10.1093/nar/gky930. - DOI - PMC - PubMed
-
- Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. Journal of Statistical Software. 2011;45:i03. doi: 10.18637/jss.v045.i03. - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- E-HKU703/18/Research Grants Council, University Grants Committee/International
- T12-708/12N/Research Grants Council, University Grants Committee/International
- AoE/M-04/04/Research Grants Council, University Grants Committee/International
- ‘973’ 2014CB942900/Ministry of Science and Technology of the People's Republic of China/International
- FDN-148408/CIHR/Canada
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

