Dysregulation of sonic hedgehog signaling causes hearing loss in ciliopathy mouse models
- PMID: 33382037
- PMCID: PMC7806262
- DOI: 10.7554/eLife.56551
Dysregulation of sonic hedgehog signaling causes hearing loss in ciliopathy mouse models
Abstract
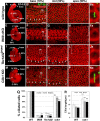
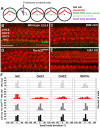
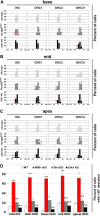
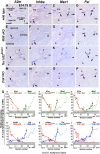
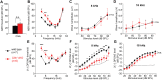
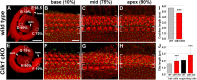
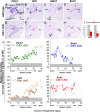
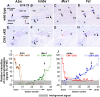
Defective primary cilia cause a range of diseases known as ciliopathies, including hearing loss. The etiology of hearing loss in ciliopathies, however, remains unclear. We analyzed cochleae from three ciliopathy mouse models exhibiting different ciliogenesis defects: Intraflagellar transport 88 (Ift88), Tbc1d32 (a.k.a. bromi), and Cilk1 (a.k.a. Ick) mutants. These mutants showed multiple developmental defects including shortened cochlear duct and abnormal apical patterning of the organ of Corti. Although ciliogenic defects in cochlear hair cells such as misalignment of the kinocilium are often associated with the planar cell polarity pathway, our results showed that inner ear defects in these mutants are primarily due to loss of sonic hedgehog signaling. Furthermore, an inner ear-specific deletion of Cilk1 elicits low-frequency hearing loss attributable to cellular changes in apical cochlear identity that is dedicated to low-frequency sound detection. This type of hearing loss may account for hearing deficits in some patients with ciliopathies.
Keywords: ciliopathies; developmental biology; hearing loss; mouse; primary cilia; sonic hedgehog.
© 2020, Moon et al.
Conflict of interest statement
KM, JM, HM, HK, HK, HK, JB No competing interests declared
Figures
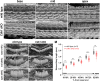
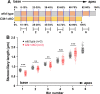
Similar articles
-
Ick Ciliary Kinase Is Essential for Planar Cell Polarity Formation in Inner Ear Hair Cells and Hearing Function.J Neurosci. 2017 Feb 22;37(8):2073-2085. doi: 10.1523/JNEUROSCI.3067-16.2017. Epub 2017 Jan 23. J Neurosci. 2017. PMID: 28115485 Free PMC article.
-
Activation of sonic hedgehog signaling by a Smoothened agonist restores congenital defects in mouse models of endocrine-cerebro-osteodysplasia syndrome.EBioMedicine. 2019 Nov;49:305-317. doi: 10.1016/j.ebiom.2019.10.016. Epub 2019 Oct 26. EBioMedicine. 2019. PMID: 31662288 Free PMC article.
-
Evaluation of Planar-Cell-Polarity Phenotypes in Ciliopathy Mouse Mutant Cochlea.J Vis Exp. 2016 Feb 21;(108):53559. doi: 10.3791/53559. J Vis Exp. 2016. PMID: 26966880 Free PMC article.
-
Ciliogenesis associated kinase 1: targets and functions in various organ systems.FEBS Lett. 2019 Nov;593(21):2990-3002. doi: 10.1002/1873-3468.13600. Epub 2019 Sep 20. FEBS Lett. 2019. PMID: 31506943 Free PMC article. Review.
-
CPLANE Complex and Ciliopathies.Biomolecules. 2022 Jun 17;12(6):847. doi: 10.3390/biom12060847. Biomolecules. 2022. PMID: 35740972 Free PMC article. Review.
Cited by
-
The morphological and functional diversity of apical microvilli.J Anat. 2023 Mar;242(3):327-353. doi: 10.1111/joa.13781. Epub 2022 Oct 25. J Anat. 2023. PMID: 36281951 Free PMC article. Review.
-
Hedgehog signaling regulates Wolffian duct development through the primary cilium†.Biol Reprod. 2023 Feb 13;108(2):241-257. doi: 10.1093/biolre/ioac210. Biol Reprod. 2023. PMID: 36525341 Free PMC article.
-
Position Specific Alternative Splicing and Gene Expression Profiles Along the Tonotopic Axis of Chick Cochlea.Front Mol Biosci. 2021 Sep 8;8:726976. doi: 10.3389/fmolb.2021.726976. eCollection 2021. Front Mol Biosci. 2021. PMID: 34568429 Free PMC article.
-
The Kinocilia of Cochlear Hair Cells: Structures, Functions, and Diseases.Front Cell Dev Biol. 2021 Aug 5;9:715037. doi: 10.3389/fcell.2021.715037. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34422834 Free PMC article. Review.
-
The multifaceted links between hearing loss and chronic kidney disease.Nat Rev Nephrol. 2024 May;20(5):295-312. doi: 10.1038/s41581-024-00808-2. Epub 2024 Jan 29. Nat Rev Nephrol. 2024. PMID: 38287134 Review.
References
-
- Abdelhamed ZA, Natarajan S, Wheway G, Inglehearn CF, Toomes C, Johnson CA, Jagger DJ. The Meckel-Gruber syndrome protein TMEM67 controls basal body positioning and epithelial branching morphogenesis in mice via the non-canonical wnt pathway. Disease Models & Mechanisms. 2015;8:527–541. doi: 10.1242/dmm.019083. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

