Electroosmotic flow: From microfluidics to nanofluidics
- PMID: 33382088
- PMCID: PMC8247933
- DOI: 10.1002/elps.202000313
Electroosmotic flow: From microfluidics to nanofluidics
Abstract
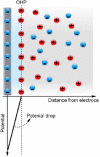
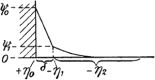
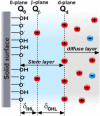
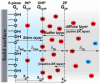
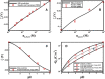
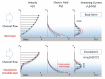
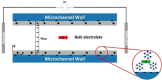
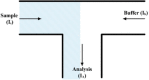
Electroosmotic flow (EOF), a consequence of an imposed electric field onto an electrolyte solution in the tangential direction of a charged surface, has emerged as an important phenomenon in electrokinetic transport at the micro/nanoscale. Because of their ability to efficiently pump liquids in miniaturized systems without incorporating any mechanical parts, electroosmotic methods for fluid pumping have been adopted in versatile applications-from biotechnology to environmental science. To understand the electrokinetic pumping mechanism, it is crucial to identify the role of an ionically polarized layer, the so-called electrical double layer (EDL), which forms in the vicinity of a charged solid-liquid interface, as well as the characteristic length scale of the conducting media. Therefore, in this tutorial review, we summarize the development of electrical double layer models from a historical point of view to elucidate the interplay and configuration of water molecules and ions in the vicinity of a solid-liquid interface. Moreover, we discuss the physicochemical phenomena owing to the interaction of electrical double layer when the characteristic length of the conducting media is decreased from the microscale to the nanoscale. Finally, we highlight the pioneering studies and the most recent works on electro osmotic flow devoted to both theoretical and experimental aspects.
Keywords: Electrical double layer / Electro osmosis / Microchannels / Nanochannels / Porous media.
© 2020 The Authors. Electrophoresis published by Wiley-VCH GmbH.
Conflict of interest statement
Figures























Similar articles
-
Numerical analysis of field-modulated electroosmotic flows in microchannels with arbitrary numbers and configurations of discrete electrodes.Biomed Microdevices. 2010 Dec;12(6):959-66. doi: 10.1007/s10544-010-9450-1. Biomed Microdevices. 2010. PMID: 20668948
-
Effects of the Electric Double Layer Characteristic and Electroosmotic Regulation on the Tribological Performance of Water-Based Cutting Fluids.Micromachines (Basel). 2023 Oct 31;14(11):2029. doi: 10.3390/mi14112029. Micromachines (Basel). 2023. PMID: 38004886 Free PMC article.
-
Electrokinetic transport phenomena in nanofluidics and their applications.Electrophoresis. 2023 Dec;44(23):1756-1773. doi: 10.1002/elps.202300115. Epub 2023 Jul 12. Electrophoresis. 2023. PMID: 37438973 Review.
-
On the Bipolar DC Flow Field-Effect-Transistor for Multifunctional Sample Handing in Microfluidics: A Theoretical Analysis under the Debye⁻Huckel Limit.Micromachines (Basel). 2018 Feb 16;9(2):82. doi: 10.3390/mi9020082. Micromachines (Basel). 2018. PMID: 30393361 Free PMC article.
-
The role of electroosmotic flow in transdermal iontophoresis.Adv Drug Deliv Rev. 2001 Mar 1;46(1-3):281-305. doi: 10.1016/s0169-409x(00)00138-1. Adv Drug Deliv Rev. 2001. PMID: 11259844 Review.
Cited by
-
Investigation of induced electroosmotic flow in small-scale capillary electrophoresis devices: Strategies for control and reversal.Electrophoresis. 2024 Oct;45(19-20):1764-1774. doi: 10.1002/elps.202400107. Epub 2024 Jul 25. Electrophoresis. 2024. PMID: 39054801
-
Tuning and Coupling Irreversible Electroosmotic Water Flow in Ionic Diodes: Methylation of an Intrinsically Microporous Polyamine (PIM-EA-TB).ACS Appl Mater Interfaces. 2023 Sep 13;15(36):42369-42377. doi: 10.1021/acsami.3c10220. Epub 2023 Aug 28. ACS Appl Mater Interfaces. 2023. PMID: 37638824 Free PMC article.
-
Current status and future application of electrically controlled micro/nanorobots in biomedicine.Front Bioeng Biotechnol. 2024 Jan 19;12:1353660. doi: 10.3389/fbioe.2024.1353660. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38314349 Free PMC article. Review.
-
Connected Droplet Shape Analysis for Nanoflow Quantification in Thin Electroosmotic Micropumps and a Tunable Convex Lens Application.Langmuir. 2023 Feb 21;39(7):2569-2578. doi: 10.1021/acs.langmuir.2c02875. Epub 2023 Feb 10. Langmuir. 2023. PMID: 36763988 Free PMC article.
-
Periodic Flows in Microfluidics.Small. 2024 Dec;20(50):e2404685. doi: 10.1002/smll.202404685. Epub 2024 Sep 9. Small. 2024. PMID: 39246195 Free PMC article. Review.
References
-
- Overbeek, J. Th. G. , Wijga, P. W. O. , Recl. Trav. Chim. Pays‐Bas 1946, 65, 556–563.
-
- Burgreen, D. , Nakache, F. R. , J. Phys. Chem. 1964, 68, 1084–1091.
-
- Rice, C. L. , Whitehead, R. , J. Phys. Chem. 1965, 69, 4017–4024.
-
- Pretorius, V. , Hopkins, B. J. , Schieke, J. D. , J. Chromatogr 1974, 99, 23.
-
- Levine, S. , Marriott, J. R. , Neale, G. , Epstein, N. , J. Colloid Interface Sci. 1975, 52, 136–149.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

