Antihypertensive effects of exercise involve reshaping of gut microbiota and improvement of gut-brain axis in spontaneously hypertensive rat
- PMID: 33382364
- PMCID: PMC7781639
- DOI: 10.1080/19490976.2020.1854642
Antihypertensive effects of exercise involve reshaping of gut microbiota and improvement of gut-brain axis in spontaneously hypertensive rat
Abstract
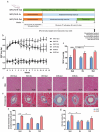
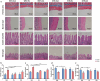
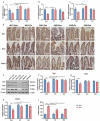
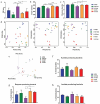
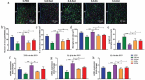
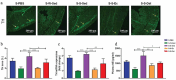
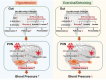
Exercise (Ex) has long been recognized to produce beneficial effects on hypertension (HTN). This coupled with evidence of gut dysbiosis and an impaired gut-brain axis led us to hypothesize that reshaping of gut microbiota and improvement in impaired gut-brain axis would, in part, be associated with beneficial influence of exercise. Male spontaneously hypertensive rats (SHR) and Wistar Kyoto (WKY) rats were randomized into sedentary, trained, and detrained groups. Trained rats underwent moderate-intensity exercise for 12 weeks, whereas, detrained groups underwent 8 weeks of moderate-intensity exercise followed by 4 weeks of detraining. Fecal microbiota, gut pathology, intestinal inflammation, and permeability, brain microglia and neuroinflammation were analyzed. We observed that exercise training resulted in a persistent decrease in systolic blood pressure in the SHR. This was associated with increase in microbial α diversity, altered β diversity, and enrichment of beneficial bacterial genera. Furthermore, decrease in the number of activated microglia, neuroinflammation in the hypothalamic paraventricular nucleus, improved gut pathology, inflammation, and permeability were also observed in the SHR following exercise. Interestingly, short-term detraining did not abolish these exercise-mediated improvements. Finally, fecal microbiota transplantation from exercised SHR into sedentary SHR resulted in attenuated SBP and an improved gut-brain axis. These observations support our concept that an impaired gut-brain axis is linked to HTN and exercise ameliorates this impairment to induce antihypertensive effects.
Keywords: Exercise; gut-brain axis; hypertension; microbiota; microglia.
Conflict of interest statement
All authors confirm that they are no conflicts of interest.
Figures




Similar articles
-
Curcumin ameliorates hypertension via gut-brain communication in spontaneously hypertensive rat.Toxicol Appl Pharmacol. 2021 Oct 15;429:115701. doi: 10.1016/j.taap.2021.115701. Epub 2021 Aug 25. Toxicol Appl Pharmacol. 2021. PMID: 34453990
-
Sustained Captopril-Induced Reduction in Blood Pressure Is Associated With Alterations in Gut-Brain Axis in the Spontaneously Hypertensive Rat.J Am Heart Assoc. 2019 Feb 19;8(4):e010721. doi: 10.1161/JAHA.118.010721. J Am Heart Assoc. 2019. PMID: 30755073 Free PMC article.
-
Microglial Cells Impact Gut Microbiota and Gut Pathology in Angiotensin II-Induced Hypertension.Circ Res. 2019 Mar;124(5):727-736. doi: 10.1161/CIRCRESAHA.118.313882. Circ Res. 2019. PMID: 30612527 Free PMC article.
-
Microbiome and substances of abuse.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Mar 8;105:110113. doi: 10.1016/j.pnpbp.2020.110113. Epub 2020 Sep 22. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 32971216 Review.
-
Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer's disease.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Mar 2;106:110112. doi: 10.1016/j.pnpbp.2020.110112. Epub 2020 Sep 16. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 32949638 Review.
Cited by
-
Exercise Intervention for Alzheimer's Disease: Unraveling Neurobiological Mechanisms and Assessing Effects.Life (Basel). 2023 Nov 30;13(12):2285. doi: 10.3390/life13122285. Life (Basel). 2023. PMID: 38137886 Free PMC article. Review.
-
Impact of exercise training on gut microbiome imbalance in obese individuals: a study based on Mendelian randomization analysis.Front Physiol. 2024 Jan 3;14:1264931. doi: 10.3389/fphys.2023.1264931. eCollection 2023. Front Physiol. 2024. PMID: 38235382 Free PMC article.
-
Hexokinase 2 Promotes Cell Proliferation and Tumor Formation through the Wnt/β-catenin Pathway-mediated Cyclin D1/c-myc Upregulation in Epithelial Ovarian Cancer.J Cancer. 2022 May 13;13(8):2559-2569. doi: 10.7150/jca.71894. eCollection 2022. J Cancer. 2022. PMID: 35711830 Free PMC article.
-
Mechanisms Leading to Increased Insulin-Stimulated Cerebral Glucose Uptake in Obesity and Insulin Resistance: A High-Fat Diet and Exercise Training Intervention PET Study with Rats (CROSRAT).J Funct Morphol Kinesiol. 2024 Mar 25;9(2):58. doi: 10.3390/jfmk9020058. J Funct Morphol Kinesiol. 2024. PMID: 38651416 Free PMC article.
-
Study on the mechanism of acupuncture to improve mild cognitive impairment in hypertension by regulating intestinal microbiome.Front Neurosci. 2024 Dec 23;18:1495384. doi: 10.3389/fnins.2024.1495384. eCollection 2024. Front Neurosci. 2024. PMID: 39764388 Free PMC article. Review.
References
-
- Toral M, Robles-Vera I, de la Visitacion N, Romero M, Sanchez M, Gomez-Guzman M, Rodriguez-Nogales A, Yang T, Jimenez R, Algieri F, et al. Role of the immune system in vascular function and blood pressure control induced by faecal microbiota transplantation in rats. Acta Physiol (Oxf). 2019;227:e13285. doi: 10.1111/apha.13285. - DOI - PubMed
-
- Toral M, Robles-Vera I, de la Visitacion N, Romero M, Yang T, Sanchez M, Gomez-Guzman M, Jimenez R, Raizada MK, Duarte J, et al. Critical role of the interaction gut microbiota - sympathetic nervous system in the regulation of blood pressure. Front Physiol. 2019;10:231. doi: 10.3389/fphys.2019.00231. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
