Technical Note: A fast and monolithic prototype clinical proton radiography system optimized for pencil beam scanning
- PMID: 33382453
- PMCID: PMC7965348
- DOI: 10.1002/mp.14700
Technical Note: A fast and monolithic prototype clinical proton radiography system optimized for pencil beam scanning
Abstract
Purpose: To demonstrate a proton-imaging system based on well-established fast scintillator technology to achieve high performance with low cost and complexity, with the potential of a straightforward translation into clinical use.
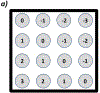
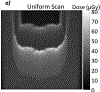
Methods: The system tracks individual protons through one (X, Y) scintillating fiber tracker plane upstream and downstream of the object and into a 13-cm -thick scintillating block residual energy detector. The fibers in the tracker planes are multiplexed into silicon photomultipliers (SiPMs) to reduce the number of electronics channels. The light signal from the residual energy detector is collected by 16 photomultiplier tubes (PMTs). Only four signals from the PMTs are output from each event, which allows for fast signal readout. A robust calibration method of the PMT signal to residual energy has been developed to obtain accurate proton images. The development of patient-specific scan patterns using multiple input energies allows for an image to be produced with minimal excess dose delivered to the patient.
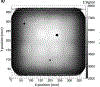
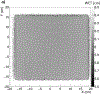
Results: The calibration of signals in the energy detector produces accurate residual range measurements limited by intrinsic range straggling. We measured the water-equivalent thickness (WET) of a block of solid water (physical thickness of 6.10 mm) with a proton radiograph. The mean WET from all pixels in the block was 6.13 cm (SD 0.02 cm). The use of patient-specific scan patterns using multiple input energies enables imaging with a compact range detector.
Conclusions: We have developed a prototype clinical proton radiography system for pretreatment imaging in proton radiation therapy. We have optimized the system for use with pencil beam scanning systems and have achieved a reduction of size and complexity compared to previous designs.
Keywords: calibration; proton imaging; proton radiography.
© 2020 American Association of Physicists in Medicine.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors have intellectual property rights to the innovations described in this paper. James S. Welsh has served as a medical advisor to ProTom International. Don F. DeJongh and Victor Rykalin are co-owners of ProtonVDA LLC.
Figures












References
-
- Romero JL, Osborne JH, Brady FP, et al. Patient positioning for proton therapy using a proton range telescope. Nucl Inst Methods Phys Res A. 1995;356(2–3):558–565. doi:10.1016/0168-9002(94)01353-5 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

