Relmacabtagene autoleucel (relma-cel) CD19 CAR-T therapy for adults with heavily pretreated relapsed/refractory large B-cell lymphoma in China
- PMID: 33382529
- PMCID: PMC7897944
- DOI: 10.1002/cam4.3686
Relmacabtagene autoleucel (relma-cel) CD19 CAR-T therapy for adults with heavily pretreated relapsed/refractory large B-cell lymphoma in China
Abstract
Background: Despite numerous chimeric antigen receptor T-cell (CAR-T) trials conducted in China, no CAR-T has been registered in the country. Furthermore, China law and regulations restrict the export of patient material for CAR-T manufacture abroad. Relma-cel (JWCAR029), an anti-CD19 product produced with a commercial-ready process in China, was evaluated in the first prospective, single-arm, multicenter, pivotal study of CAR-T therapy conducted under Chinese IND to support an NMPA-accepted BLA submission in relapsed/refractory (r/r) LBCL (NCT04089215).
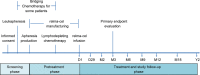
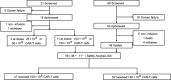
Methods: Patients were randomized to receive either 100 × 106 (low dose, n = 27) or 150 × 106 (high dose, n = 32) CAR+ T-cells as a single infusion following lymphodepleting chemotherapy (fludarabine 25 mg/m2 and cyclophosphamide 250 mg/m2 daily × 3), and then, monitored for efficacy and safety outcomes and pharmacokinetics. The primary endpoint was ORR at 3 months, as assessed by the investigators. Secondary endpoints included DOR, PFS, OS, and adverse event frequency/severity and cell expansion kinetics.
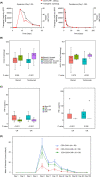
Results: As of the data cutoff on 17 June 2020, 68 patients were enrolled, and 59 were treated. Among the 58 efficacy-evaluable patients, the primary endpoint of 3 month ORR was 60.3% (95% CI, 46.6-73.0), excluding the null hypothesis rate of 20%. Any grade and severe grade CRS occurred in 47.5% and 5.1%, respectively, and any grade and severe grade neurotoxicity events occurred in 20.3% and 5.1%.
Conclusions: Relma-cel met the primary endpoint analysis and demonstrated a high rate of durable responses and low rate of CAR-T-associated toxicities in patients with r/r LBCL in a multicenter trial supporting regulatory submission in China.
Keywords: CAR-T; CD19; LBCL; Relma-cel; cellular kinetics.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
No conflict of interest disclosures from authors.
Figures


References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B‐cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2015;26(Suppl 5):v116‐v125. - PubMed
-
- Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem‐cell transplantation for relapsed and refractory aggressive lymphomas: NCIC‐CTG LY.12. J Clin Oncol. 2014;32:3490‐3496. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

