Performance of an Antigen-Based Test for Asymptomatic and Symptomatic SARS-CoV-2 Testing at Two University Campuses - Wisconsin, September-October 2020
- PMID: 33382679
- PMCID: PMC9191905
- DOI: 10.15585/mmwr.mm695152a3
Performance of an Antigen-Based Test for Asymptomatic and Symptomatic SARS-CoV-2 Testing at Two University Campuses - Wisconsin, September-October 2020
Abstract
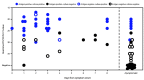
Antigen-based tests for SARS-CoV-2, the virus that causes coronavirus disease 2019 (COVID-19), are inexpensive and can return results within 15 minutes (1). Antigen tests have received Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for use in asymptomatic and symptomatic persons within the first 5-12 days after symptom onset (2). These tests have been used at U.S. colleges and universities and other congregate settings (e.g., nursing homes and correctional and detention facilities), where serial testing of asymptomatic persons might facilitate early case identification (3-5). However, test performance data from symptomatic and asymptomatic persons are limited. This investigation evaluated performance of the Sofia SARS Antigen Fluorescent Immunoassay (FIA) (Quidel Corporation) compared with real-time reverse transcription-polymerase chain reaction (RT-PCR) for SARS-CoV-2 detection among asymptomatic and symptomatic persons at two universities in Wisconsin. During September 28-October 9, a total of 1,098 paired nasal swabs were tested using the Sofia SARS Antigen FIA and real-time RT-PCR. Virus culture was attempted on all antigen-positive or real-time RT-PCR-positive specimens. Among 871 (79%) paired swabs from asymptomatic participants, the antigen test sensitivity was 41.2%, specificity was 98.4%, and in this population the estimated positive predictive value (PPV) was 33.3%, and negative predictive value (NPV) was 98.8%. Antigen test performance was improved among 227 (21%) paired swabs from participants who reported one or more symptoms at specimen collection (sensitivity = 80.0%; specificity = 98.9%; PPV = 94.1%; NPV = 95.9%). Virus was isolated from 34 (46.6%) of 73 antigen-positive or real-time RT-PCR-positive nasal swab specimens, including two of 18 that were antigen-negative and real-time RT-PCR-positive (false-negatives). The advantages of antigen tests such as low cost and rapid turnaround might allow for rapid identification of infectious persons. However, these advantages need to be balanced against lower sensitivity and lower PPV, especially among asymptomatic persons. Confirmatory testing with an FDA-authorized nucleic acid amplification test (NAAT), such as RT-PCR, should be considered after negative antigen test results in symptomatic persons, and after positive antigen test results in asymptomatic persons (1).
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- CDC. Coronavirus disease 2019 (COVID-19): interim guidance for antigen testing for SARS-CoV-2. Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-gu...
-
- Food and Drug Administration. In vitro diagnostics EUAs. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2020. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em...
-
- CDC. Coronavirus disease 2019 (COVID-19): guidance for testing, screening, and outbreak response for institutions of higher education (IHEs). Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://www.cdc.gov/coronavirus/2019-ncov/community/colleges-universitie...
-
- CDC. Coronavirus disease 2019 (COVID-19): testing guidelines for nursing homes. Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-homes-testing.html
-
- CDC. Coronavirus disease 2019 (COVID-19): interim considerations for SARS-CoV-2 testing in correctional and detention facilities. Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://www.cdc.gov/coronavirus/2019-ncov/community/correction-detention...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

