Prognostic roles of diabetes mellitus and hypertension in advanced hepatocellular carcinoma treated with sorafenib
- PMID: 33382703
- PMCID: PMC7775090
- DOI: 10.1371/journal.pone.0244293
Prognostic roles of diabetes mellitus and hypertension in advanced hepatocellular carcinoma treated with sorafenib
Abstract
Background & aims: It remains limited whether diabetes mellitus (DM) and hypertension (HTN) affect the prognosis of advanced hepatocellular carcinoma (HCC) treated with sorafenib. Our study attempted to elucidate the roles of DM/HTN and the effects of diabetes medications among advanced HCC patients receiving sorafenib.
Methods: From August 2012 to February 2018, 733 advanced HCC patients receiving sorafenib were enrolled at China Medical University, Taichung, Taiwan. According to the presence/absence of DM or HTN, they were divided into four groups: control [DM(-)/HTN(-), n = 353], DM-only [DM(+)/HTN(-), n = 91], HTN-only [DM(-)/HTN(+), n = 184] and DM+HTN groups [DM(+)/HTN(+), n = 105]. Based on the types of diabetes medications, there were three groups among DM patients (the combined cohort of DM-only and DM+HTN groups), including metformin (n = 63), non-metformin oral hypoglycemic agent (OHA) (n = 104) and regular insulin (RI)/neutral protamine hagedorn (NPH) groups (n = 29). We then assessed the survival differences between these groups.
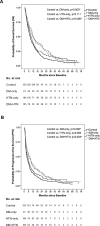
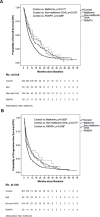
Results: DM-only and DM+HTN groups significantly presented longer overall survival (OS) than control group (control vs. DM-only, 7.70 vs. 11.83 months, p = 0.003; control vs. DM+HTN, 7.70 vs. 11.43 months, p = 0.008). However, there was no significant OS difference between control and HTN-only group (7.70 vs. 8.80 months, p = 0.111). Besides, all groups of DM patients showed significantly longer OS than control group (control vs. metformin, 7.70 vs. 12.60 months, p = 0.011; control vs. non-metformin OHA, 7.70 vs. 10.80 months, p = 0.016; control vs. RI/NPH, 7.70 vs. 15.20 months, p = 0.026).
Conclusions: Rather than HTN, DM predicts better prognosis in advanced HCC treated with sorafenib. Besides, metformin, non-metformin OHA and RI/NPH are associated with longer survival among DM-related advanced HCC patients receiving sorafenib.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: Validation study and biological rationale.Eur J Cancer. 2017 Nov;86:106-114. doi: 10.1016/j.ejca.2017.09.003. Epub 2017 Oct 3. Eur J Cancer. 2017. PMID: 28985579
-
Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib.Expert Opin Pharmacother. 2015;16(18):2719-25. doi: 10.1517/14656566.2015.1102887. Epub 2015 Oct 29. Expert Opin Pharmacother. 2015. PMID: 26513009
-
Treatment with metformin is associated with a prolonged survival in patients with hepatocellular carcinoma.Liver Int. 2019 Apr;39(4):714-726. doi: 10.1111/liv.14048. Epub 2019 Feb 7. Liver Int. 2019. PMID: 30663219
-
Diabetes and hepatocellular carcinoma: A pathophysiological link and pharmacological management.Biomed Pharmacother. 2018 Oct;106:991-1002. doi: 10.1016/j.biopha.2018.06.095. Epub 2018 Jul 14. Biomed Pharmacother. 2018. PMID: 30119271 Review.
-
A single center experience of sorafenib in advanced hepatocellular carcinoma patients: evaluation of prognostic factors.Eur J Gastroenterol Hepatol. 2011 Nov;23(12):1233-8. doi: 10.1097/MEG.0b013e32834bd2d0. Eur J Gastroenterol Hepatol. 2011. PMID: 21941188 Review.
Cited by
-
Immune cell dynamics and the impact on the efficiency of transvascular antitumor interventional therapies in hepatocellular carcinoma patients.Front Immunol. 2024 Oct 8;15:1450525. doi: 10.3389/fimmu.2024.1450525. eCollection 2024. Front Immunol. 2024. PMID: 39439786 Free PMC article.
-
Does Type 2 Diabetes Increase the Risk of Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease Patients? A Systematic Review.Cureus. 2023 Mar 13;15(3):e36079. doi: 10.7759/cureus.36079. eCollection 2023 Mar. Cureus. 2023. PMID: 37065332 Free PMC article. Review.
-
Clinicopathological Aspects and Inflammation-Immune Markers in Alcohol and/or Hepatitis C Virus-Induced Hepatocellular Carcinoma Patients Treated With Sorafenib.Gastroenterology Res. 2024 Feb;17(1):23-31. doi: 10.14740/gr1689. Epub 2024 Feb 28. Gastroenterology Res. 2024. PMID: 38463146 Free PMC article.
-
The Emerging Role of Metformin in the Treatment of Hepatocellular Carcinoma: Is There Any Value in Repurposing Metformin for HCC Immunotherapy?Cancers (Basel). 2023 Jun 13;15(12):3161. doi: 10.3390/cancers15123161. Cancers (Basel). 2023. PMID: 37370771 Free PMC article. Review.
-
Sorafenib with or without co-interventions for hepatocellular carcinoma.Cochrane Database Syst Rev. 2025 Jun 26;6(6):CD015851. doi: 10.1002/14651858.CD015851. Cochrane Database Syst Rev. 2025. PMID: 40568833 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

