Antibody response to SARS-CoV-2 infection in humans: A systematic review
- PMID: 33382764
- PMCID: PMC7775097
- DOI: 10.1371/journal.pone.0244126
Antibody response to SARS-CoV-2 infection in humans: A systematic review
Abstract
Background: Progress in characterising the humoral immune response to Severe Acute Respiratory Syndrome 2 (SARS-CoV-2) has been rapid but areas of uncertainty persist. Assessment of the full range of evidence generated to date to understand the characteristics of the antibody response, its dynamics over time, its determinants and the immunity it confers will have a range of clinical and policy implications for this novel pathogen. This review comprehensively evaluated evidence describing the antibody response to SARS-CoV-2 published from 01/01/2020-26/06/2020.
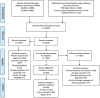
Methods: Systematic review. Keyword-structured searches were carried out in MEDLINE, Embase and COVID-19 Primer. Articles were independently screened on title, abstract and full text by two researchers, with arbitration of disagreements. Data were double-extracted into a pre-designed template, and studies critically appraised using a modified version of the Public Health Ontario Meta-tool for Quality Appraisal of Public Health Evidence (MetaQAT) tool, with resolution of disagreements by consensus. Findings were narratively synthesised.
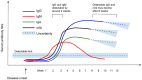
Results: 150 papers were included. Most studies (113 or 75%) were observational in design, were based wholly or primarily on data from hospitalised patients (108, 72%) and had important methodological limitations. Few considered mild or asymptomatic infection. Antibody dynamics were well described in the acute phase, up to around three months from disease onset, but the picture regarding correlates of the antibody response was inconsistent. IgM was consistently detected before IgG in included studies, peaking at weeks two to five and declining over a further three to five weeks post-symptom onset depending on the patient group; IgG peaked around weeks three to seven post-symptom onset then plateaued, generally persisting for at least eight weeks. Neutralising antibodies were detectable within seven to 15 days following disease onset, with levels increasing until days 14-22 before levelling and then decreasing, but titres were lower in those with asymptomatic or clinically mild disease. Specific and potent neutralising antibodies have been isolated from convalescent plasma. Cross-reactivity but limited cross-neutralisation with other human coronaviridae was reported. Evidence for protective immunity in vivo was limited to small, short-term animal studies, showing promising initial results in the immediate recovery phase.
Conclusions: Literature on antibody responses to SARS-CoV-2 is of variable quality with considerable heterogeneity of methods, study participants, outcomes measured and assays used. Although acute phase antibody dynamics are well described, longer-term patterns are much less well evidenced. Comprehensive assessment of the role of demographic characteristics and disease severity on antibody responses is needed. Initial findings of low neutralising antibody titres and possible waning of titres over time may have implications for sero-surveillance and disease control policy, although further evidence is needed. The detection of potent neutralising antibodies in convalescent plasma is important in the context of development of therapeutics and vaccines. Due to limitations with the existing evidence base, large, cross-national cohort studies using appropriate statistical analysis and standardised serological assays and clinical classifications should be prioritised.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; JM is chief scientific officer, shareholder and scientific founder of Leucid Bio, a spinout company focused on development of cellular therapeutic agents; no other relationships or activities that could appear to have influenced the submitted work. This does not alter our adherence to PLoS ONE policies on sharing data and materials.
Figures

References
-
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard. 2020. https://covid19.who.int/
-
- Davies NG, Kucharski AJ, Eggo RM, Gimma A, Group CC-19 W, Edmunds WJ. The effect of non-pharmaceutical interventions on COVID-19 cases, deaths and demand for hospital services in the UK: a modelling study. medRxiv [Preprint]. 2020;2020.04.01.20049908. 10.1016/S2468-2667(20)30133-X - DOI - PMC - PubMed
-
- WHO. “Immunity passports” in the context of COVID-19. WHO—Sci Br. 2020;
-
- Tiberghien P, de Lambalerie X, Morel P, Gallian P, Lacombe K, Yazdanpanah Y. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how. Vox Sang. 2020;02:2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

