Lack of elevated pre-ART elastase-ANCA levels in patients developing TB-IRIS
- PMID: 33382831
- PMCID: PMC7775098
- DOI: 10.1371/journal.pone.0244800
Lack of elevated pre-ART elastase-ANCA levels in patients developing TB-IRIS
Abstract
Background: Tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) in HIV-TB co-infected patients receiving antiretroviral therapy (ART) has been linked to neutrophil activation. Anti-neutrophil cytoplasmic antibodies (ANCAs) are also associated with neutrophil activation. Since ANCAs are reportedly skewed in TB and HIV infections, we investigated plasma levels of 7 ANCAs in TB-IRIS patients.
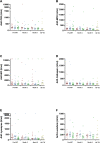
Methods: We retrospectively compared 17 HIV-TB patients who developed TB-IRIS with controls of similar CD4 count, age and gender who did not (HIV+TB+ n = 17), HIV-infected patients without TB (HIV+TB-, n = 17) and 10 HIV-negative (HIV-TB-) controls. Frozen plasma was collected before ART, at 3 and 9 months of ART, and examined by ELISA for levels of 7 ANCAs directed against; Proteinase 3 (PR3), Myeloperoxidase (MPO), Permeability-increasing protein (BPI), Elastase, Cathepsin, Lysozyme, and Lactoferrin.
Results: Compared to HIV+TB+ controls, pre-ART anti-elastase levels were lower in TB-IRIS patients (p = 0.026) and HIV-TB- controls (p = 0.044), whereas other ANCAs did not show significant differences between groups at any time point. A significant decrease over time could be observed in TB-IRIS patients during ART for anti -PR3 (p = 0.027), -lysozyme (p = 0.011), and -lactoferrin (p = 0.019). Conversely, HIV+TB+ controls showed a significant decrease over time for anti -MPO (p = 0.002), -lyzosyme (p = 0.002) and -elastase (p < 0.001).
Conclusion: The lack of elevated anti-elastase levels in TB-IRIS patients as opposed to HIV+TB+ controls correspond to previous findings of lowered immune capacity in patients that will develop TB-IRIS. This may suggest a specific role for anti-elastase, elastase or even matrix-metalloproteinases in TB-IRIS. The precise dynamics of neutrophil activation in HIV-TB merits further investigation and could provide more insight in the early mechanisms leading up to TB-IRIS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Burman W, Weis S, Vernon A, Khan A, Benator D, Jones B, et al. Frequency, severity and duration of immune reconstitution events in HIV-related tuberculosis SUMMARY. 2007;11: 1282–1289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

