Enteral broad-spectrum antibiotics antagonize the effect of fecal microbiota transplantation in preterm pigs
- PMID: 33382952
- PMCID: PMC7781584
- DOI: 10.1080/19490976.2020.1849997
Enteral broad-spectrum antibiotics antagonize the effect of fecal microbiota transplantation in preterm pigs
Abstract
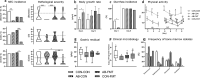
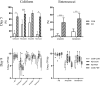
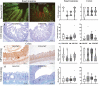
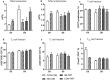
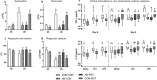
Preterm infants are at risk of multiple morbidities including necrotizing enterocolitis (NEC). Suspected NEC patients receive intravenous antibiotics (AB) to prevent sepsis, although enteral AB is arguably more effective at reducing NEC but is rarely used due to the risk of AB resistance. Fecal microbiota transplantation (FMT) has shown protective effects against NEC in animal experiments, but the interaction between AB and FMT has not been investigated in neonates. We hypothesized that administration of enteral AB followed by rectal FMT would effectively prevent NEC with negligible changes in AB resistance and systemic immunity. Using preterm piglets, we examined host and gut microbiota responses to AB, FMT, or a sequential combination thereof, with emphasis on NEC development. In a saline-controlled experiment, preterm piglets (n = 67) received oro-gastric neomycin (50 mg/kg/d) and amoxicillin-clavulanate (50/12.5 mg/kg/d) (hereafter AB) for four days after cesarean delivery, and were subsequently given rectal FMT from healthy suckling piglet donors. Whereas AB protected the stomach and small intestine, and FMT primarily protected the colon, the sequential combination treatment surprisingly provided no NEC protection. Furthermore, minor changes in the gut microbiota composition were observed in response to either treatment, although AB treatment decreased species diversity and increased AB resistance among coliform bacteria and Enterococci, which were both partly reversed by FMT. Besides, enteral AB treatment suppressed cellular and functional systemic immune development, which was not prevented by subsequent FMT. We discovered an antagonistic relationship between enteral AB and FMT in terms of NEC development. The outcome may depend on choice of AB compounds, FMT composition, doses, treatment duration, and administration routes, but these results challenge the applicability of enteral AB and FMT in preterm infants.
Keywords: Gut microbiota; antibiotics; antibiotics resistance; fecal microbiota transplantation; immunity; necrotizing enterocolitis; prematurity.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
