Increased demand for NAD+ relative to ATP drives aerobic glycolysis
- PMID: 33382985
- PMCID: PMC8315838
- DOI: 10.1016/j.molcel.2020.12.012
Increased demand for NAD+ relative to ATP drives aerobic glycolysis
Abstract
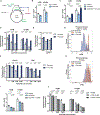
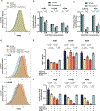
Aerobic glycolysis, or preferential fermentation of glucose-derived pyruvate to lactate despite available oxygen, is associated with proliferation across many organisms and conditions. To better understand that association, we examined the metabolic consequence of activating the pyruvate dehydrogenase complex (PDH) to increase pyruvate oxidation at the expense of fermentation. We find that increasing PDH activity impairs cell proliferation by reducing the NAD+/NADH ratio. This change in NAD+/NADH is caused by increased mitochondrial membrane potential that impairs mitochondrial electron transport and NAD+ regeneration. Uncoupling respiration from ATP synthesis or increasing ATP hydrolysis restores NAD+/NADH homeostasis and proliferation even when glucose oxidation is increased. These data suggest that when demand for NAD+ to support oxidation reactions exceeds the rate of ATP turnover in cells, NAD+ regeneration by mitochondrial respiration becomes constrained, promoting fermentation, despite available oxygen. This argues that cells engage in aerobic glycolysis when the demand for NAD+ is in excess of the demand for ATP.
Keywords: Aerobic Glycolysis; Cell Metabolism; Fermentation; NAD+; PDK; Warburg Effect.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests; however, M.G.V.H. discloses he is on the advisory board of Molecular Cell and is a scientific advisor for Agios Pharmaceuticals, Aeglea Biotherapeutics, Auron Therapeutics, Faeth Therapeutics, and iTeos Therapeutics. S.S. is member of the scientific advisory board of Arcus Biosciences, Venn Therapeutics, Tango Therapeutics, and Replimune and serves as a scientific advisor for Dragonfly Therapeutics, Merck, Ribon, Torque, and TAKEDA. A.L. is a current employee of a Flagship Pioneering biotechnology start-up company.
Figures



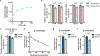
Comment in
-
A time to build and a time to burn: glucose metabolism for every season.Mol Cell. 2021 Feb 18;81(4):642-644. doi: 10.1016/j.molcel.2021.02.003. Mol Cell. 2021. PMID: 33606971
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

