Cellular hallmarks of aging emerge in the ovary prior to primordial follicle depletion
- PMID: 33383072
- PMCID: PMC8279026
- DOI: 10.1016/j.mad.2020.111425
Cellular hallmarks of aging emerge in the ovary prior to primordial follicle depletion
Abstract
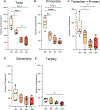
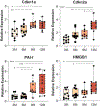
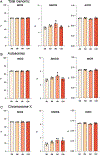
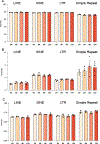
Decline in ovarian reserve with advancing age is associated with reduced fertility and the emergence of metabolic disturbances, osteoporosis, and neurodegeneration. Recent studies have provided insight into connections between ovarian insufficiency and systemic aging, although the basic mechanisms that promote ovarian reserve depletion remain unknown. Here, we sought to determine if chronological age is linked to changes in ovarian cellular senescence, transcriptomic, and epigenetic mechanisms in a mouse model. Histological assessments and transcriptional analyses revealed the accumulation of lipofuscin aggresomes and senescence-related transcripts (Cdkn1a, Cdkn2a, Pai-1 and Hmgb1) significantly increased with advancing age. Transcriptomic profiling and pathway analyses following RNA sequencing, revealed an upregulation of genes related to pro-inflammatory stress and cell-cycle inhibition, whereas genes involved in cell-cycle progression were downregulated; which could be indicative of senescent cell accumulation. The emergence of these senescence-related markers preceded the dramatic decline in primordial follicle reserve observed. Whole Genome Oxidative Bisulfite Sequencing (WGoxBS) found no genome-wide or genomic context-specific DNA methylation and hydroxymethylation changes with advancing age. These findings suggest that cellular senescence may contribute to ovarian aging, and thus, declines in ovarian follicular reserve. Cell-type-specific analyses across the reproductive lifespan are needed to fully elucidate the mechanisms that promote ovarian insufficiency.
Keywords: Aging; Cellular senescence; DNA methylation; Epigenetics; Ovary.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
DECLARATION OF INTEREST
The authors have no conflicts of interest to declare.
Figures
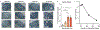

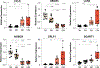

References
-
- Adriaens I, Smitz J, Jacquet P, 2009. The current knowledge on radiosensitivity of ovarian follicle development stages. Human reproduction update 15, 359–377. - PubMed
-
- Baird DT, 1974. The endocrinology of ovarian steroid secretion. Eur J Obstet Gynecol Reprod Biol 4, 31–39. - PubMed
-
- Baker TG, 1963. A Quantitative and Cytological Study of Germ Cells in Human Ovaries. Proc R Soc Lond B Biol Sci 158, 417–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

