Germline and Tumor Sequencing as a Diagnostic Tool To Resolve Suspected Lynch Syndrome
- PMID: 33383211
- PMCID: PMC7927277
- DOI: 10.1016/j.jmoldx.2020.12.003
Germline and Tumor Sequencing as a Diagnostic Tool To Resolve Suspected Lynch Syndrome
Abstract
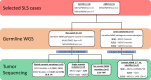
Patients in whom mismatch repair (MMR)-deficient cancer develops in the absence of pathogenic variants of germline MMR genes or somatic hypermethylation of the MLH1 gene promoter are classified as having suspected Lynch syndrome (SLS). Germline whole-genome sequencing (WGS) and targeted and genome-wide tumor sequencing were applied to identify the underlying cause of tumor MMR deficiency in SLS. Germline WGS was performed on samples from 14 cancer-affected patients with SLS, including two sets of first-degree relatives. MMR genes were assessed for germline pathogenic variants, including complex structural rearrangements and noncoding variants. Tumor tissue was assessed for somatic MMR gene mutations using targeted, whole-exome sequencing or WGS. Germline WGS identified pathogenic MMR variants in 3 of the 14 cases (21.4%), including a 9.5-megabase inversion disrupting MSH2 in a mother and daughter. Excluding these 3 MMR carriers, tumor sequencing identified at least two somatic MMR gene mutations in 8 of 11 tumors tested (72.7%). In a second mother-daughter pair, a somatic cause of tumor MMR deficiency was supported by the presence of double somatic MSH2 mutations in their respective tumors. More than 70% of SLS cases had double somatic MMR mutations in the absence of germline pathogenic variants in the MMR or other DNA repair-related genes on WGS, and, therefore, were confidently assigned a noninherited cause of tumor MMR deficiency.
Copyright © 2021 Association for Molecular Pathology and American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Tumor testing to identify lynch syndrome in two Australian colorectal cancer cohorts.J Gastroenterol Hepatol. 2017 Feb;32(2):427-438. doi: 10.1111/jgh.13468. J Gastroenterol Hepatol. 2017. PMID: 27273229 Free PMC article.
-
A tumor focused approach to resolving the etiology of DNA mismatch repair deficient tumors classified as suspected Lynch syndrome.J Transl Med. 2023 Apr 26;21(1):282. doi: 10.1186/s12967-023-04143-1. J Transl Med. 2023. PMID: 37101184 Free PMC article.
-
Whole Gene Capture Analysis of 15 CRC Susceptibility Genes in Suspected Lynch Syndrome Patients.PLoS One. 2016 Jun 14;11(6):e0157381. doi: 10.1371/journal.pone.0157381. eCollection 2016. PLoS One. 2016. PMID: 27300758 Free PMC article.
-
Lynch syndrome and Lynch syndrome mimics: The growing complex landscape of hereditary colon cancer.World J Gastroenterol. 2015 Aug 21;21(31):9253-61. doi: 10.3748/wjg.v21.i31.9253. World J Gastroenterol. 2015. PMID: 26309352 Free PMC article. Review.
-
Muir-Torre Syndrome and founder mismatch repair gene mutations: A long gone historical genetic challenge.Gene. 2016 Sep 10;589(2):127-32. doi: 10.1016/j.gene.2015.06.078. Epub 2015 Jul 2. Gene. 2016. PMID: 26143115 Review.
Cited by
-
Canadian consensus for the assessment and testing of Lynch syndrome.J Med Genet. 2025 Apr 17;62(5):326-334. doi: 10.1136/jmg-2024-110465. J Med Genet. 2025. PMID: 40081873 Free PMC article.
-
Unexplained mismatch repair deficiency: Case closed.HGG Adv. 2022 Dec 14;4(1):100167. doi: 10.1016/j.xhgg.2022.100167. eCollection 2023 Jan 12. HGG Adv. 2022. PMID: 36624813 Free PMC article.
-
"Left in limbo": Exploring how patients with colorectal cancer interpret and respond to a suspected Lynch syndrome diagnosis.Hered Cancer Clin Pract. 2021 Oct 16;19(1):43. doi: 10.1186/s13053-021-00201-1. Hered Cancer Clin Pract. 2021. PMID: 34656160 Free PMC article.
-
Intratumoral presence of the genotoxic gut bacteria pks+ E. coli, Enterotoxigenic Bacteroides fragilis, and Fusobacterium nucleatum and their association with clinicopathological and molecular features of colorectal cancer.Br J Cancer. 2024 Mar;130(5):728-740. doi: 10.1038/s41416-023-02554-x. Epub 2024 Jan 10. Br J Cancer. 2024. PMID: 38200234 Free PMC article.
-
The intersection of homologous recombination (HR) and mismatch repair (MMR) pathways in DNA repair-defective tumors.NPJ Precis Oncol. 2024 Sep 5;8(1):190. doi: 10.1038/s41698-024-00672-0. NPJ Precis Oncol. 2024. PMID: 39237751 Free PMC article. Review.
References
-
- Ligtenberg M.J., Kuiper R.P., Chan T.L., Goossens M., Hebeda K.M., Voorendt M., Lee T.Y., Bodmer D., Hoenselaar E., Hendriks-Cornelissen S.J., Tsui W.Y., Kong C.K., Brunner H.G., van Kessel A.G., Yuen S.T., van Krieken J.H., Leung S.Y., Hoogerbrugge N. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3' exons of TACSTD1. Nat Genet. 2009;41:112–117. - PubMed
-
- Win A.K., Young J.P., Lindor N.M., Tucker K.M., Ahnen D.J., Young G.P., Buchanan D.D., Clendenning M., Giles G.G., Winship I., Macrae F.A., Goldblatt J., Southey M.C., Arnold J., Thibodeau S.N., Gunawardena S.R., Bapat B., Baron J.A., Casey G., Gallinger S., Le Marchand L., Newcomb P.A., Haile R.W., Hopper J.L., Jenkins M.A. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol. 2012;30:958–964. - PMC - PubMed
-
- Thibodeau S.N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. - PubMed
-
- Klarskov L., Ladelund S., Holck S., Roenlund K., Lindebjerg J., Elebro J., Halvarsson B., von Salome J., Bernstein I., Nilbert M. Interobserver variability in the evaluation of mismatch repair protein immunostaining. Hum Pathol. 2010;41:1387–1396. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

