Bipolar androgen therapy sensitizes castration-resistant prostate cancer to subsequent androgen receptor ablative therapy
- PMID: 33383350
- PMCID: PMC9844588
- DOI: 10.1016/j.ejca.2020.11.043
Bipolar androgen therapy sensitizes castration-resistant prostate cancer to subsequent androgen receptor ablative therapy
Abstract
Background: Cyclical, high-dose testosterone administration, termed bipolar androgen therapy (BAT), can induce clinical responses and restore sensitivity to androgen signalling inhibition in patients with previously treated castration-resistant prostate cancer (PCa) (CRPC). This trial evaluated whether BAT is a safe and effective first-line hormonal therapy for patients with CRPC.
Patients and methods: In cohort C of this single-centre, open-label, phase II, multi-cohort trial (RE-sensitizing with Supraphysiologic Testosterone to Overcome REsistance study), 29 patients with CRPC received first-line hormonal therapy with 400 mg of testosterone cypionate intramuscularly every 28 days concurrent with a luteinising hormone-releasing hormone agonist/antagonist. The primary end-point of the study was the PSA50 response rate to BAT treatment.
Results: After treatment with BAT, four of 29 patients (14%; 95% confidence interval [CI]: 4-32%) experienced a PSA50 response. The median radiographic progression-free survival to BAT was 8.5 months (95% CI: 6.9-15.1) for patients with metastatic CRPC. After progression on BAT, 17 of 18 patients (94%; 95% CI: 73-100%) achieved a PSA50 response and 15 of 18 patients (83%; 95% CI: 59-96) achieved a PSA90 response on abiraterone or enzalutamide. Twelve of 15 patients (80%; 95% CI: 52-96) with metastatic CRPC remain on abiraterone or enzalutamide with a median duration of follow-up of 11.2 months.
Conclusion: As first-line hormonal treatment for CRPC, BAT was well tolerated and resulted in prolonged disease stabilisation. After progression on BAT, patients had favourable responses to second-generation androgen receptor-targeted therapy.
Trial registration: ClinicalTrials.gov NCT02090114.
Keywords: Bipolar androgen therapy; Castration-resistant prostate cancer; RESTORE trial; Testosterone.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement E.S.A. reports being a paid consultant/advisor to Janssen, Astellas, Sanofi, Dendreon, Pfizer, Amgen, Lilly, Bayer, AstraZeneca, Bristol-Myers Squibb, Clovis and Merck; reports receiving research funding to his institution from Janssen, Johnson & Johnson, Sanofi, Dendreon, Genentech, Novartis, Tokai, Bristol Myers-Squibb, AstraZeneca, Clovis, and Merck and he reports being the co-inventor of an AR-V7 biomarker technology that has been licenced to Qiagen.
Figures
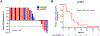
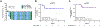
References
-
- Isaacs JT & Isaacs WB Androgen receptor outwits prostate cancer drugs. Nature medicine vol. 10 26–27 (2004). - PubMed
-
- Scher HI & Sawyers CL Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol 23, 8253–8261 (2005). - PubMed
-
- Visakorpi T et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat. Genet 9, 401–406 (1995). - PubMed
-
- Linja MJ et al. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 61, 3550–3555 (2001). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

