Treatment of Male Sexual Partners of Women With Bacterial Vaginosis: A Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 33383580
- PMCID: PMC8326574
- DOI: 10.1093/cid/ciaa1903
Treatment of Male Sexual Partners of Women With Bacterial Vaginosis: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Background: We aimed to determine if treatment of male sexual partners of women with recurrent bacterial vaginosis (BV) with oral metronidazole 2×/day for 7 days (ie, multidose metronidazole) significantly decreased BV recurrence rates in the female.
Methods: This was a multicenter, 2-arm, double-blind, placebo-controlled study. Women with recurrent BV and current diagnosis of BV by Amsel and Nugent were enrolled. Multidose metronidazole for 7 days was dispensed to women. Male partners were randomized to placebo versus multidose metronidazole for 7 days and asked to refrain from unprotected sex for 14 days. Female follow-up visits were conducted at day 21 and 8 and 16 weeks. Male follow-up visits occurred at days 14-21. BV cure was defined as 0-2 Amsel criteria and Nugent score 0-6 in the female partner with the primary endpoint at 16 weeks.
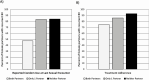
Results: 214 couples were enrolled. In the intent-to-treat population, there was no significant difference between treatment arms for the primary outcome. BV treatment failure occurred in 81% and 80% of women in the metronidazole and placebo arms through the third follow-up visit, respectively (P > .999). However, women whose male partners adhered to study medication were less likely to fail treatment (adjusted relative risk, .85; 95% CI, .73-.99; P = .035). This finding persisted in post hoc comparisons in the metronidazole arm.
Conclusions: Overall, this study did not find that male partner treatment with multidose metronidazole significantly reduces BV recurrence in female partners, although women whose partners adhered to multidose metronidazole were less likely to fail treatment.
Clinical trials registration: (NCT02209519).
Keywords: bacterial vaginosis; male partners; metronidazole; sexually transmitted infection; treatment.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Does Partner Treatment Impact on Bacterial Vaginosis Cure?Clin Infect Dis. 2021 Aug 2;73(3):e680-e682. doi: 10.1093/cid/ciaa1907. Clin Infect Dis. 2021. PMID: 33411901 No abstract available.
Comment on
-
Does Partner Treatment Impact on Bacterial Vaginosis Cure?Clin Infect Dis. 2021 Aug 2;73(3):e680-e682. doi: 10.1093/cid/ciaa1907. Clin Infect Dis. 2021. PMID: 33411901 No abstract available.
References
-
- Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983; 74:14–22. - PubMed
-
- Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis 2019; 46:304–11. - PubMed
-
- Cohen CR, Duerr A, Pruithithada N, et al. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS 1995; 9:1093–7. - PubMed
-
- Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999; 180:1863–8. - PubMed
-
- Taha TE, Gray RH, Kumwenda NI, et al. HIV infection and disturbances of vaginal flora during pregnancy. J Acquir Immune Defic Syndr Hum Retrovirol 1999; 20:52–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

