Collagen Structure-Function Mapping Informs Applications for Regenerative Medicine
- PMID: 33383610
- PMCID: PMC7824244
- DOI: 10.3390/bioengineering8010003
Collagen Structure-Function Mapping Informs Applications for Regenerative Medicine
Abstract
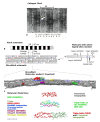
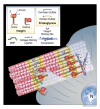
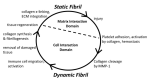
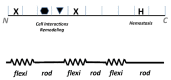
Type I collagen, the predominant protein of vertebrates, assembles into fibrils that orchestrate the form and function of bone, tendon, skin, and other tissues. Collagen plays roles in hemostasis, wound healing, angiogenesis, and biomineralization, and its dysfunction contributes to fibrosis, atherosclerosis, cancer metastasis, and brittle bone disease. To elucidate the type I collagen structure-function relationship, we constructed a type I collagen fibril interactome, including its functional sites and disease-associated mutations. When projected onto an X-ray diffraction model of the native collagen microfibril, data revealed a matrix interaction domain that assumes structural roles including collagen assembly, crosslinking, proteoglycan (PG) binding, and mineralization, and the cell interaction domain supporting dynamic aspects of collagen biology such as hemostasis, tissue remodeling, and cell adhesion. Our type III collagen interactome corroborates this model. We propose that in quiescent tissues, the fibril projects a structural face; however, tissue injury releases blood into the collagenous stroma, triggering exposure of the fibrils' cell and ligand binding sites crucial for tissue remodeling and regeneration. Applications of our research include discovery of anti-fibrotic antibodies and elucidating their interactions with collagen, and using insights from our angiogenesis studies and collagen structure-function model to inform the design of super-angiogenic collagens and collagen mimetics.
Keywords: angiogenesis; connective tissue; extracellular matrix; fibrosis; hemostasis; interactome; ligand binding; microfibril; therapeutic antibodies; type I collagen; type III collagen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates.J Biol Chem. 2008 Jul 25;283(30):21187-97. doi: 10.1074/jbc.M709319200. Epub 2008 May 15. J Biol Chem. 2008. PMID: 18487200 Free PMC article.
-
Type I collagen and collagen mimetics as angiogenesis promoting superpolymers.Curr Pharm Des. 2007;13(35):3608-21. doi: 10.2174/138161207782794176. Curr Pharm Des. 2007. PMID: 18220798 Review.
-
Mapping the heparin-binding sites on type I collagen monomers and fibrils.J Cell Biol. 1994 Jun;125(5):1179-88. doi: 10.1083/jcb.125.5.1179. J Cell Biol. 1994. PMID: 8195298 Free PMC article.
-
Collagen fibril surface displays a constellation of sites capable of promoting fibril assembly, stability, and hemostasis.Connect Tissue Res. 2011 Feb;52(1):18-24. doi: 10.3109/03008207.2010.511354. Epub 2010 Nov 30. Connect Tissue Res. 2011. PMID: 21117898 Free PMC article.
-
Collagenous Extracellular Matrix Biomaterials for Tissue Engineering: Lessons from the Common Sea Urchin Tissue.Int J Mol Sci. 2017 Apr 25;18(5):901. doi: 10.3390/ijms18050901. Int J Mol Sci. 2017. PMID: 28441344 Free PMC article. Review.
Cited by
-
Extracellular Microenvironment Alterations in Ductal Carcinoma In Situ and Invasive Breast Cancer Pathologies by Multiplexed Spatial Proteomics.Int J Mol Sci. 2024 Jun 19;25(12):6748. doi: 10.3390/ijms25126748. Int J Mol Sci. 2024. PMID: 38928454 Free PMC article.
-
Adhesion force microscopy is sensitive to the charge distribution at the surface of single collagen fibrils.Nanoscale Adv. 2022 Oct 18;4(22):4829-4837. doi: 10.1039/d2na00514j. eCollection 2022 Nov 8. Nanoscale Adv. 2022. PMID: 36381506 Free PMC article.
-
Dissecting the phenotypic variability of osteogenesis imperfecta.Dis Model Mech. 2022 May 1;15(5):dmm049398. doi: 10.1242/dmm.049398. Epub 2022 May 16. Dis Model Mech. 2022. PMID: 35575034 Free PMC article.
-
Mechanisms of reducing joint stiffness by blocking collagen fibrillogenesis in a rabbit model of posttraumatic arthrofibrosis.PLoS One. 2021 Sep 7;16(9):e0257147. doi: 10.1371/journal.pone.0257147. eCollection 2021. PLoS One. 2021. PMID: 34492074 Free PMC article.
-
Identification of a human type XVII collagen fragment with high capacity for maintaining skin health.Synth Syst Biotechnol. 2024 Jun 6;9(4):733-741. doi: 10.1016/j.synbio.2024.06.001. eCollection 2024 Dec. Synth Syst Biotechnol. 2024. PMID: 38911060 Free PMC article.
References
-
- Marini J.C., Forlino A., Cabral W.A., Barnes A.M., San Antonio J.D., Milgrom S., Hyland J.C., Korkko J., Prockop D.J., De Paepe A., et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: Regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum. Mutat. 2007;28:209–221. doi: 10.1002/humu.20429. - DOI - PMC - PubMed
-
- Piez K.A., Reddi A.H. Extracellular Matrix Biochemistry. Elsevier; New York, NY, USA: 1984. p. xvi.473p
-
- Jacenko O., Olsen B.R., LuValle P. Organization and regulation of collagen genes. Crit. Rev. Eukaryot. Gene Expr. 1991;1:327–353. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

