Current Trends and Future Prospects of Molecular Targeted Therapy in Head and Neck Squamous Cell Carcinoma
- PMID: 33383632
- PMCID: PMC7795499
- DOI: 10.3390/ijms22010240
Current Trends and Future Prospects of Molecular Targeted Therapy in Head and Neck Squamous Cell Carcinoma
Abstract
In recent years, advances in drug therapy for head and neck squamous cell carcinoma (HNSCC) have progressed rapidly. In addition to cytotoxic anti-cancer agents such as platinum-based drug (cisplatin and carboplatin) and taxane-based drugs (docetaxel and paclitaxel), epidermal growth factor receptor-tyrosine kinase inhibitors (cetuximab) and immune checkpoint inhibitors such as anti-programmed cell death-1 (PD-1) antibodies (nivolumab and pembrolizumab) have come to be used. The importance of anti-cancer drug therapy is increasing year by year. Therefore, we summarize clinical trials of molecular targeted therapy and biomarkers in HNSCC from previous studies. Here we show the current trends and future prospects of molecular targeted therapy in HNSCC.
Keywords: cetuximab; head and neck squamous cell carcinoma; immune checkpoint inhibitor; molecular targeted therapy; multi-oncogene panel test; photoimmunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
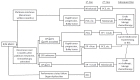
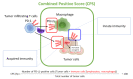
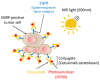
References
-
- Harrington K.J., Ferris R.L., Blumenschein G., Jr., Colevas A.D., Fayette J., Licitra L., Kasper S., Even C., E Vokes E., Worden F., et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017;18:1104–1115. doi: 10.1016/S1470-2045(17)30421-7. - DOI - PMC - PubMed
-
- Burtness B., Harrington K.J., Greil R., Soulières D., Tahara M., De Castro G., Jr., Psyrri A., Basté N., Neupane P., Bratland Å., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. - DOI - PubMed
-
- Kish J., Drelichman A., Jacobs J., Hoschner J., Kinzie J., Loh J., Weaver A., Al-Sarraf M. Clinical trial of cisplatin and 5-FU infusion as initial treatment for advanced squamous cell carcinoma of the head and neck. Cancer Treat. Rep. 1982;66:471–474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

