Fast Diffusion of the Unassembled PetC1-GFP Protein in the Cyanobacterial Thylakoid Membrane
- PMID: 33383642
- PMCID: PMC7823997
- DOI: 10.3390/life11010015
Fast Diffusion of the Unassembled PetC1-GFP Protein in the Cyanobacterial Thylakoid Membrane
Abstract
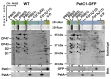
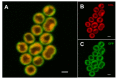
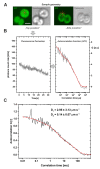
Biological membranes were originally described as a fluid mosaic with uniform distribution of proteins and lipids. Later, heterogeneous membrane areas were found in many membrane systems including cyanobacterial thylakoids. In fact, cyanobacterial pigment-protein complexes (photosystems, phycobilisomes) form a heterogeneous mosaic of thylakoid membrane microdomains (MDs) restricting protein mobility. The trafficking of membrane proteins is one of the key factors for long-term survival under stress conditions, for instance during exposure to photoinhibitory light conditions. However, the mobility of unbound 'free' proteins in thylakoid membrane is poorly characterized. In this work, we assessed the maximal diffusional ability of a small, unbound thylakoid membrane protein by semi-single molecule FCS (fluorescence correlation spectroscopy) method in the cyanobacterium Synechocystis sp. PCC6803. We utilized a GFP-tagged variant of the cytochrome b6f subunit PetC1 (PetC1-GFP), which was not assembled in the b6f complex due to the presence of the tag. Subsequent FCS measurements have identified a very fast diffusion of the PetC1-GFP protein in the thylakoid membrane (D = 0.14 - 2.95 µm2s-1). This means that the mobility of PetC1-GFP was comparable with that of free lipids and was 50-500 times higher in comparison to the mobility of proteins (e.g., IsiA, LHCII-light-harvesting complexes of PSII) naturally associated with larger thylakoid membrane complexes like photosystems. Our results thus demonstrate the ability of free thylakoid-membrane proteins to move very fast, revealing the crucial role of protein-protein interactions in the mobility restrictions for large thylakoid protein complexes.
Keywords: FCS; cyanobacteria; photosynthesis; proteins mobility; thylakoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Steinbeck J., Ross I.L., Rothnagel R., Gabelein P., Schulze S., Giles N., Ali R., Drysdale R., Sierecki E., Gambin Y., et al. Structure of a PSI-LHCI-cyt b6f supercomplex in Chlamydomonas reinhardtii promoting cyclic electron flow under anaerobic conditions. Proc. Natl. Acad. Sci. USA. 2018;115:10517–10522. doi: 10.1073/pnas.1809973115. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

