Characterization and Differentiation of the Tumor Microenvironment (TME) of Orthotopic and Subcutaneously Grown Head and Neck Squamous Cell Carcinoma (HNSCC) in Immunocompetent Mice
- PMID: 33383676
- PMCID: PMC7796118
- DOI: 10.3390/ijms22010247
Characterization and Differentiation of the Tumor Microenvironment (TME) of Orthotopic and Subcutaneously Grown Head and Neck Squamous Cell Carcinoma (HNSCC) in Immunocompetent Mice
Abstract
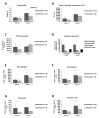
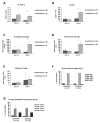
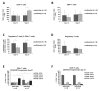
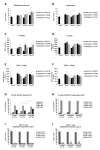
For the development and evaluation of new head and neck squamous cell carcinoma (HNSCC) therapeutics, suitable, well-characterized animal models are needed. Thus, by analyzing orthotopic versus subcutaneous models of HNSCC in immunocompetent mice, we evaluated the existence of adenosine-related immunosuppressive B- and T lymphocyte populations within the tumor microenvironment (TME). Applying the SCC VII model for the induction of HNSCC in immunocompetent C3H/HeN mice, the cellular TME was characterized after tumor initiation over time by flow cytometry. The TME in orthotopic grown tumors revealed a larger population of tumor-infiltrating lymphocytes (TIL) with more B cells and CD4+ T cells than the subcutaneously grown tumors. Immune cell populations in the blood and bone marrow showed a rather distinct reaction toward tumor induction and tumor location compared to the spleen, lymph nodes, or thymus. In addition, large numbers of immunosuppressive B- and T cells were identified within the TME but also in secondary lymphoid organs, independently of the tumor initiation site. The altered immunogenic TME may influence the response to any treatment attempt. Moreover, when analyzing the TME and other lymphoid organs of tumor-bearing mice, we observed conditions reflecting largely those of patients suffering from HNSCC suggesting the C3H/HeN mouse model as a suitable tool for studies aiming to target immunosuppression to improve anti-cancer therapies.
Keywords: CD4+ T cells; SCC VII orthotopic mouse model; SCC VII subcutaneous mouse model; adenosine-related immunosuppression; head and neck cancer; regulatory B cells; tumor microenvironment; tumor-infiltrating lymphocytes.
Conflict of interest statement
Simon Laban: Advisory Boards: Merck Sharp & Dohme (MSD), Bristol Myers Squibb (BMS), Astra Zeneca (AZ). Honoraria: MSD, BMS, AZ, Merck Serono. Johannes Doescher: Advisory Boards: Merck Serono and MSD. Honoraria: Merck Serono. Patrick J. Schuler: Advisory Boards: BMS and MSD. Thomas K. Hoffmann: Advisory Boards: MSD and BMS. Honoraria: MSD, BMS, and Merck Serono. The research was supported by the University of Ulm ‘Promotionsprogramm Experimentelle Medizin’ (M.B.). All other authors declare no conflict of interests.
Figures






Similar articles
-
Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma.Oncotarget. 2017 Jul 4;8(27):44418-44433. doi: 10.18632/oncotarget.17901. Oncotarget. 2017. PMID: 28574843 Free PMC article.
-
Identification of B Cell Subpopulations with Pro- and Anti-Tumorigenic Properties in an Immunocompetent Mouse Model of Head and Neck Squamous Cell Carcinoma.Cells. 2024 Dec 29;14(1):20. doi: 10.3390/cells14010020. Cells. 2024. PMID: 39791721 Free PMC article.
-
CBD promotes antitumor activity by modulating tumor immune microenvironment in HPV associated head and neck squamous cell carcinoma.Front Immunol. 2025 May 22;16:1528520. doi: 10.3389/fimmu.2025.1528520. eCollection 2025. Front Immunol. 2025. PMID: 40475776 Free PMC article.
-
Tertiary lymphoid structures in head and neck squamous cell carcinoma (Review).Oncol Rep. 2025 Jul;54(1):76. doi: 10.3892/or.2025.8909. Epub 2025 May 9. Oncol Rep. 2025. PMID: 40341409 Review.
-
Tumor immune microenvironment in head and neck cancers.Mol Carcinog. 2020 Jul;59(7):766-774. doi: 10.1002/mc.23162. Epub 2020 Feb 3. Mol Carcinog. 2020. PMID: 32017286 Free PMC article. Review.
Cited by
-
Tumor Regression upon Intratumoral and Subcutaneous Dosing of the STING Agonist ALG-031048 in Mouse Efficacy Models.Int J Mol Sci. 2023 Nov 13;24(22):16274. doi: 10.3390/ijms242216274. Int J Mol Sci. 2023. PMID: 38003463 Free PMC article.
-
The impact of tumor immunogenicity on cancer pain phenotype using syngeneic oral cancer mouse models.Front Pain Res (Lausanne). 2022 Sep 12;3:991725. doi: 10.3389/fpain.2022.991725. eCollection 2022. Front Pain Res (Lausanne). 2022. PMID: 36172037 Free PMC article.
-
Expanding the role of combined immunochemotherapy and immunoradiotherapy in the management of head and neck cancer (Review).Oncol Lett. 2023 Jul 17;26(3):372. doi: 10.3892/ol.2023.13958. eCollection 2023 Sep. Oncol Lett. 2023. PMID: 37965160 Free PMC article. Review.
-
Immunotherapy against glioblastoma using backpack-activated neutrophils.Bioeng Transl Med. 2024 Aug 13;10(1):e10712. doi: 10.1002/btm2.10712. eCollection 2025 Jan. Bioeng Transl Med. 2024. PMID: 39801750 Free PMC article.
-
Aerobic exercise training mitigates tumor growth and cancer-induced splenomegaly through modulation of non-platelet platelet factor 4 expression.Sci Rep. 2023 Dec 11;13(1):21970. doi: 10.1038/s41598-023-47217-2. Sci Rep. 2023. PMID: 38081853 Free PMC article.
References
-
- Marur S., Forastiere A.A. Proceedings of the Mayo Clinic Proceedings. Volume 83. Elsevier; Amsterdam, The Netherlands: 2008. Head and neck cancer: Changing epidemiology, diagnosis, and treatment; pp. 489–501. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

