Redox-Responsive Nanocarrier for Controlled Release of Drugs in Inflammatory Skin Diseases
- PMID: 33383706
- PMCID: PMC7823658
- DOI: 10.3390/pharmaceutics13010037
Redox-Responsive Nanocarrier for Controlled Release of Drugs in Inflammatory Skin Diseases
Abstract
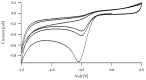
A synthetic route for redox-sensitive and non-sensitive core multi-shell (CMS) carriers with sizes below 20 nm and narrow molecular weight distributions was established. Cyclic voltammetric measurements were conducted characterizing the redox potentials of reduction-sensitive CMS while showcasing its reducibility through glutathione and tris(2-carboxyethyl)-phosphine as a proof of concept. Measurements of reduction-initiated release of the model dye Nile red by time-dependent fluorescence spectroscopy showed a pronounced release for the redox-sensitive CMS nanocarrier (up to 90% within 24 h) while the non-sensitive nanocarriers showed no release in PBS. Penetration experiments using ex vivo human skin showed that the redox-sensitive CMS nanocarrier could deliver higher percentages of the loaded macrocyclic dye meso-tetra (m-hydroxyphenyl) porphyrin (mTHPP) to the skin as compared to the non-sensitive CMS nanocarrier. Encapsulation experiments showed that these CMS nanocarriers can encapsulate dyes or drugs with different molecular weights and hydrophobicity. A drug content of 1 to 6 wt% was achieved for the anti-inflammatory drugs dexamethasone and rapamycin as well as fluorescent dyes such as Nile red and porphyrins. These results show that redox-initiated drug release is a promising strategy to improve the topical drug delivery of macrolide drugs.
Keywords: CMS nanocarriers; anti-inflammatory drugs; cyclic voltammetry; dexamethasone; disulfide; rapamycin; redox; skin penetration; stimuli responsive.
Conflict of interest statement
The authors declare no conflict of interest. The funding is project based; yet the funders had no role in the design, in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Topical Delivery of Rapamycin by Means of Microenvironment-Sensitive Core-Multi-Shell Nanocarriers: Assessment of Anti-Inflammatory Activity in an ex vivo Skin/T Cell Co-Culture Model.Int J Nanomedicine. 2021 Oct 22;16:7137-7151. doi: 10.2147/IJN.S330716. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34712046 Free PMC article.
-
Oxidation-Sensitive Core-Multishell Nanocarriers for the Controlled Delivery of Hydrophobic Drugs.ACS Biomater Sci Eng. 2021 Jun 14;7(6):2485-2495. doi: 10.1021/acsbiomaterials.0c01771. Epub 2021 Apr 27. ACS Biomater Sci Eng. 2021. PMID: 33905661
-
Core-multishell nanocarriers: Transport and release of dexamethasone probed by soft X-ray spectromicroscopy.J Control Release. 2016 Nov 28;242:64-70. doi: 10.1016/j.jconrel.2016.08.028. Epub 2016 Aug 24. J Control Release. 2016. PMID: 27568290
-
Redox Nano-Architectures: Perspectives and Implications in Diagnosis and Treatment of Human Diseases.Antioxid Redox Signal. 2019 Feb 10;30(5):762-785. doi: 10.1089/ars.2017.7412. Epub 2018 Mar 2. Antioxid Redox Signal. 2019. PMID: 29334759 Review.
-
Photo-Induced Drug Release from Polymeric Micelles and Liposomes: Phototriggering Mechanisms in Drug Delivery Systems.Polymers (Basel). 2022 Mar 23;14(7):1286. doi: 10.3390/polym14071286. Polymers (Basel). 2022. PMID: 35406160 Free PMC article. Review.
Cited by
-
Novel Adhesive Nanocarriers Based on Mussel-Inspired Polyglycerols for the Application onto Mucosal Tissues.Pharmaceutics. 2022 Apr 26;14(5):940. doi: 10.3390/pharmaceutics14050940. Pharmaceutics. 2022. PMID: 35631526 Free PMC article.
-
Biodegradable and Stimuli-Responsive Nanomaterials for Targeted Drug Delivery in Autoimmune Diseases.J Funct Biomater. 2025 Jan 14;16(1):24. doi: 10.3390/jfb16010024. J Funct Biomater. 2025. PMID: 39852580 Free PMC article. Review.
-
Topical calcineurin and mammalian target of rapamycin inhibitors in inflammatory dermatoses: Current challenges and nanotechnology‑based prospects (Review).Int J Mol Med. 2024 Oct;54(4):85. doi: 10.3892/ijmm.2024.5409. Epub 2024 Aug 12. Int J Mol Med. 2024. PMID: 39129316 Free PMC article. Review.
-
Versatile and Controlled Synthesis of Degradable, Water-Soluble Bottlebrush Polymers with Poly(disulfide) Backbones Derived from α-Lipoic Acid.ACS Macro Lett. 2025 Feb 18;14(2):207-213. doi: 10.1021/acsmacrolett.4c00839. Epub 2025 Feb 3. ACS Macro Lett. 2025. PMID: 39899736 Free PMC article.
-
Topical Delivery of Rapamycin by Means of Microenvironment-Sensitive Core-Multi-Shell Nanocarriers: Assessment of Anti-Inflammatory Activity in an ex vivo Skin/T Cell Co-Culture Model.Int J Nanomedicine. 2021 Oct 22;16:7137-7151. doi: 10.2147/IJN.S330716. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34712046 Free PMC article.
References
-
- Saeidpour S., Lohan S., Anske M., Unbehauen M., Fleige E., Haag R., Meinke M., Bittl R., Teutloff C. Localization of dexamethasone within dendritic core-multishell (CMS) nanoparticles and skin penetration properties studied by multi-frequency electron paramagnetic resonance (EPR) spectroscopy. Eur. J. Pharm. Biopharm. 2017;116:94–101. doi: 10.1016/j.ejpb.2016.10.001. - DOI - PubMed
-
- Pickard C., Louafi F., McGuire C., Lowings K., Kumar P., Cooper H., Dearman R.J., Cumberbatch M., Kimber I., Healy E., et al. The Cutaneous Biochemical Redox Barrier: A Component of the Innate Immune Defenses against Sensitization by Highly Reactive Environmental Xenobiotics. J. Immunol. 2009;183:7576–7584. doi: 10.4049/jimmunol.0901064. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

