CD8+ T Cells in Atherosclerosis
- PMID: 33383733
- PMCID: PMC7823404
- DOI: 10.3390/cells10010037
CD8+ T Cells in Atherosclerosis
Abstract
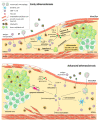
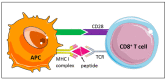
Atherosclerotic lesions are populated by cells of the innate and adaptive immune system, including CD8+ T cells. The CD8+ T cell infiltrate has recently been characterized in mouse and human atherosclerosis and revealed activated, cytotoxic, and possibly dysfunctional and exhausted cell phenotypes. In mouse models of atherosclerosis, antibody-mediated depletion of CD8+ T cells ameliorates atherosclerosis. CD8+ T cells control monopoiesis and macrophage accumulation in early atherosclerosis. In addition, CD8+ T cells exert cytotoxic functions in atherosclerotic plaques and contribute to macrophage cell death and necrotic core formation. CD8+ T cell activation may be antigen-specific, and epitopes of atherosclerosis-relevant antigens may be targets of CD8+ T cells and their cytotoxic activity. CD8+ T cell functions are tightly controlled by costimulatory and coinhibitory immune checkpoints. Subsets of regulatory CD25+CD8+ T cells with immunosuppressive functions can inhibit atherosclerosis. Importantly, local cytotoxic CD8+ T cell responses may trigger endothelial damage and plaque erosion in acute coronary syndromes. Understanding the complex role of CD8+ T cells in atherosclerosis may pave the way for defining novel treatment approaches in atherosclerosis. In this review article, we discuss these aspects, highlighting the emerging and critical role of CD8+ T cells in atherosclerosis.
Keywords: CD8+ T cells; atherosclerosis; checkpoint inhibitors; cytotoxic T cells; immunotherapy; inflammation; single cell RNA sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

