Sex and Exposure to Postnatal Chlorpyrifos Influence the Epigenetics of Feeding-Related Genes in a Transgenic APOE Mouse Model: Long-Term Implications on Body Weight after a High-Fat Diet
- PMID: 33383760
- PMCID: PMC7795072
- DOI: 10.3390/ijerph18010184
Sex and Exposure to Postnatal Chlorpyrifos Influence the Epigenetics of Feeding-Related Genes in a Transgenic APOE Mouse Model: Long-Term Implications on Body Weight after a High-Fat Diet
Abstract
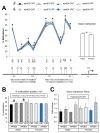
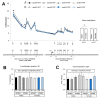
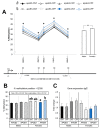
Developmental exposure to toxicants and diet can interact with an individual's genetics and produce long-lasting metabolic adaptations. The different isoforms of the apolipoprotein E (APOE) are an important source of variability in metabolic disorders and influence the response to the pesticide chlorpyrifos (CPF). We aimed to study the epigenetic regulation on feeding control genes and the influence of postnatal CPF exposure, APOE genotype, and sex, and how these modifications impact on the metabolic response to a high-fat diet (HFD). Both male and female apoE3- and apoE4-TR mice were exposed to CPF on postnatal days 10-15. The DNA methylation pattern of proopiomelanocortin, neuropeptide Y, leptin receptor, and insulin-like growth factor 2 was studied in the hypothalamus. At adulthood, the mice were given a HFD for eight weeks. The results highlight the importance of sex in the epigenetic regulation and the implication of CPF treatment and APOE genotype. The body weight progression exhibited sex-dimorphic differences, apoE4-TR males being the most susceptible to the effects induced by CPF and HFD. Overall, these results underscore the pivotal role of sex, APOE genotype, and developmental exposure to CPF on subsequent metabolic disturbances later in life and show that sex is a key variable in epigenetic regulation.
Keywords: APOE; chlorpyrifos; epigenetics; feeding control; high-fat diet.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Exposure to chlorpyrifos at different ages triggers APOE genotype-specific responses in social behavior, body weight and hypothalamic gene expression.Environ Res. 2019 Nov;178:108684. doi: 10.1016/j.envres.2019.108684. Epub 2019 Aug 23. Environ Res. 2019. PMID: 31472362
-
Learning, memory and the expression of cholinergic components in mice are modulated by the pesticide chlorpyrifos depending upon age at exposure and apolipoprotein E (APOE) genotype.Arch Toxicol. 2019 Mar;93(3):693-707. doi: 10.1007/s00204-019-02387-9. Epub 2019 Jan 18. Arch Toxicol. 2019. PMID: 30656380
-
APOE genotype and postnatal chlorpyrifos exposure modulate gut microbiota and cerebral short-chain fatty acids in preweaning mice.Food Chem Toxicol. 2020 Jan;135:110872. doi: 10.1016/j.fct.2019.110872. Epub 2019 Oct 14. Food Chem Toxicol. 2020. PMID: 31622728
-
Gestational/perinatal chlorpyrifos exposure is not associated with autistic-like behaviors in rodents.Crit Rev Toxicol. 2014 Jul;44(6):523-34. doi: 10.3109/10408444.2014.907772. Epub 2014 May 27. Crit Rev Toxicol. 2014. PMID: 24861450 Review.
-
Chlorpyrifos Induces Metabolic Disruption by Altering Levels of Reproductive Hormones.J Agric Food Chem. 2019 Sep 25;67(38):10553-10562. doi: 10.1021/acs.jafc.9b03602. Epub 2019 Sep 16. J Agric Food Chem. 2019. PMID: 31490076 Review.
Cited by
-
Impact of Endocrine Disrupting Pesticide Use on Obesity: A Systematic Review.Biomedicines. 2024 Nov 24;12(12):2677. doi: 10.3390/biomedicines12122677. Biomedicines. 2024. PMID: 39767584 Free PMC article. Review.
-
Obesity II: Establishing causal links between chemical exposures and obesity.Biochem Pharmacol. 2022 May;199:115015. doi: 10.1016/j.bcp.2022.115015. Epub 2022 Apr 5. Biochem Pharmacol. 2022. PMID: 35395240 Free PMC article. Review.
-
λ-cyhalothrin induced sex-specific inflammation, glia activation and GABAergic interneuron disruption in the hippocampus of rats.BMC Pharmacol Toxicol. 2025 Jan 29;26(1):22. doi: 10.1186/s40360-025-00860-z. BMC Pharmacol Toxicol. 2025. PMID: 39881343 Free PMC article.
-
Epigenetic disruptions in the offspring hypothalamus in response to maternal infection.Gene. 2024 Jun 5;910:148329. doi: 10.1016/j.gene.2024.148329. Epub 2024 Feb 29. Gene. 2024. PMID: 38431234 Free PMC article.
References
-
- Grandjean P., Barouki R., Bellinger D.C., Casteleyn L., Chadwick L.H., Cordier S., Etzel R.A., Gray K.A., Ha E.H., Junien C., et al. Life-long implications of developmental exposure to environmental stressors: New perspectives. Endocrinology. 2015;156:3408–3415. doi: 10.1210/en.2015-1350. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

