Histopathological Chromogranin A-Positivity Is Associated with Right-Sided Colorectal Cancers and Worse Prognosis
- PMID: 33383764
- PMCID: PMC7796394
- DOI: 10.3390/cancers13010067
Histopathological Chromogranin A-Positivity Is Associated with Right-Sided Colorectal Cancers and Worse Prognosis
Abstract
Background: Colorectal cancer (CRC) is known to be affected by paraneoplastic thrombocytosis and chromogranin A-positive neuroendocrine-cell differentiation (CgA+). Their combined effect has never been previously investigated.
Methods: A prospective cohort pilot study of 42 CRC patients and 42 age- and sex-matched controls was carried out. Plasma interleukin-6, thrombopoietin, and serum chromogranin A and -B were measured; furthermore, tumor tissue was immunohistochemically stained for CgA+.
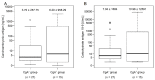
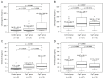
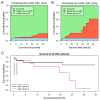
Results: Twenty-seven and 15 patients were assigned to the chromogranin A-negative (CgA-) and CgA+ groups, respectively. Within the CgA+ group, right-sided tumors were more frequent (18.5% vs. 53.3%), no stage I cancer was found, and patients of this group were in worse general condition. Compared to control subjects, chromogranin A level was higher in the CgA+ group (p = 0.0086), thrombopoietin (p = 0.0040) and chromogranin B (p = 0.0070) in the CgA- group, while interleukin-6 was high in both tumor groups (p ≤ 0.0090). Survival was significantly worse in the CgA+ group (hazard ratio: 5.73; p = 0.0378).
Conclusions: Different thrombopoietin levels indicated distinct thrombocytosis types. Within the two CRC groups, serum levels of chromogranins changed in different directions suggesting two well-distinguishable pathophysiologies. Based on these observations we propose a new subtype of CRC, which can be characterized by chromogranin A-positive neuroendocrine-cell differentiation.
Keywords: chromogranin A; chromogranin B; colorectal neoplasms; interleukin-6; neuroendocrine cells; survival analysis; thrombocytosis; thrombopoietin.
Conflict of interest statement
The authors declare no conflict of interest. The funding bodies have no role in the design of the study; collection, analysis, and interpretation of data; or in writing the manuscript.
Figures


Similar articles
-
Neuroendocrine differentiation in colorectal carcinomas.Eur J Gastroenterol Hepatol. 1995 Jul;7(7):667-74. Eur J Gastroenterol Hepatol. 1995. PMID: 8590163
-
Neuroendocrine differentiation in stage D2 prostate cancers.Int J Urol. 2008 May;15(5):423-8. doi: 10.1111/j.1442-2042.2008.02015.x. Int J Urol. 2008. PMID: 18452460
-
The prognostic significance of circulating neuroendocrine markers chromogranin a, pro-gastrin-releasing peptide and neuron-specific enolase in patients with advanced non-small-cell lung cancer.Tumour Biol. 2006;27(1):8-16. doi: 10.1159/000090151. Epub 2005 Dec 8. Tumour Biol. 2006. PMID: 16340245
-
Potential clinical value of circulating chromogranin A in patients with prostate carcinoma.Ann Oncol. 2001;12 Suppl 2:S153-7. doi: 10.1093/annonc/12.suppl_2.s153. Ann Oncol. 2001. PMID: 11762344 Review.
-
Chromogranin A: its clinical value as marker of neuroendocrine tumours.Eur J Clin Invest. 1998 Jun;28(6):431-40. doi: 10.1046/j.1365-2362.1998.00305.x. Eur J Clin Invest. 1998. PMID: 9693933 Review.
Cited by
-
Neuroendocrine differentiation: a risk fellow in colorectal cancer.World J Surg Oncol. 2023 Mar 10;21(1):89. doi: 10.1186/s12957-023-02952-8. World J Surg Oncol. 2023. PMID: 36899368 Free PMC article.
-
Prognostic significance of the aberrant expression of neuroendocrine markers in melanomas.Diagn Pathol. 2021 Aug 28;16(1):78. doi: 10.1186/s13000-021-01135-x. Diagn Pathol. 2021. PMID: 34454530 Free PMC article.
-
Neuroendocrine differentiation and serotonin expression in oesophageal adenocarcinomas after neoadjuvant therapy: correlation with clinicopathological features and outcome.Histopathology. 2025 Mar;86(4):559-570. doi: 10.1111/his.15364. Epub 2024 Nov 11. Histopathology. 2025. PMID: 39526928 Free PMC article.
-
Prediction of hereditary nonpolyposis colorectal cancer using mRNA MSH2 quantitative and the correlation with nonmodifiable factor.World J Gastrointest Pathophysiol. 2021 Nov 22;12(6):134-146. doi: 10.4291/wjgp.v12.i6.134. World J Gastrointest Pathophysiol. 2021. PMID: 34877027 Free PMC article.
-
Holistic exploration of CHGA and hsa-miR-137 in colorectal cancer via multi-omic data Integration.Heliyon. 2024 Mar 3;10(5):e27046. doi: 10.1016/j.heliyon.2024.e27046. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38495181 Free PMC article.
References
-
- National Institute of Oncology National Cancer Registry: Cancer Statistics Reports for Hungary. [(accessed on 10 June 2020)]; Available online: http://stat.nrr.hu/
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

