MixInYeast: A Multicenter Study on Mixed Yeast Infections
- PMID: 33383783
- PMCID: PMC7823447
- DOI: 10.3390/jof7010013
MixInYeast: A Multicenter Study on Mixed Yeast Infections
Abstract
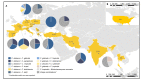
Invasive candidiasis remains one of the most prevalent systemic mycoses, and several studies have documented the presence of mixed yeast (MY) infections. Here, we describe the epidemiology, clinical, and microbiological characteristics of MY infections causing invasive candidiasis in a multicenter prospective study. Thirty-four centers from 14 countries participated. Samples were collected in each center between April to September 2018, and they were sent to a reference center to confirm identification by sequencing methods and to perform antifungal susceptibility testing, according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST). A total of 6895 yeast cultures were identified and MY occurred in 150 cases (2.2%). Europe accounted for the highest number of centers, with an overall MY rate of 4.2% (118 out of 2840 yeast cultures). Of 122 MY cases, the most frequent combinations were Candida albicans/C. glabrata (42, 34.4%), C. albicans/C. parapsilosis (17, 14%), and C. glabrata/C. tropicalis (8, 6.5%). All Candida isolates were susceptible to amphotericin B, 6.4% were fluconazole-resistant, and two isolates (1.6%) were echinocandin-resistant. Accurate identification of the species involved in MY infections is essential to guide treatment decisions.
Keywords: Candida; chrome agar; invasive candidiasis; mix infections; polymicrobial infections; yeast.
Conflict of interest statement
S.A.A. has received speaker honoraria or travel grant from Astellas, Gilead, and Pfizer in the last five years outside the submitted work. A.A.-I. has received research grants or honoraria as a speaker or advisor from Astellas, Gilead Sciences, MSD, Pfizer, F2G, Amplyx and Scynexis in the last five years outside the submitted work. MH has received research funding from Gilead and Pfizer. ER has received research grants from Astellas, Gilead, Merck and Pfizer Inc.; he is a scientific advisor and member of speaker bureaus for Astellas, Gilead, Merck and Pfizer Inc. The rest of the authors none to declare.
Figures
References
-
- Pappas P.G., Kauffman C.A., Andes D.R., Clancy C.J., Marr K.A., Ostrosky-Zeichner L., Reboli A.C., Schuster M.G., Vazquez J.A., Walsh T.J., et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015;62:e1–e50. doi: 10.1093/cid/civ933. - DOI - PMC - PubMed
-
- Pfaller M.A., Andes D.R., Diekema D.J., Horn D.L., Reboli A.C., Rotstein C., Franks B., Azie N.E. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: Data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS ONE. 2014;9:e0101510. doi: 10.1371/journal.pone.0101510. - DOI - PMC - PubMed

