Shoot Regeneration Is Not a Single Cell Event
- PMID: 33383798
- PMCID: PMC7823732
- DOI: 10.3390/plants10010058
Shoot Regeneration Is Not a Single Cell Event
Abstract
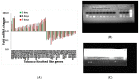
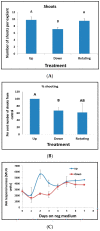
Shoot regeneration is a key tool of modern plant biotechnology. While many researchers use this process empirically, very little is known about the early molecular genetic factors and signaling events that lead to shoot regeneration. Using tobacco as a model system, we found that the inductive events required for shoot regeneration occur in the first 4-5 days following incubation on regeneration medium. Leaf segments placed on regeneration medium did not produce shoots if removed from the medium before four days indicating this time frame is crucial for the induction of shoot regeneration. Leaf segments placed on regeneration medium for longer than five days maintain the capacity to produce shoots when removed from the regeneration medium. Analysis of gene expression during the early days of incubation on regeneration medium revealed many changes occurring with no single expression pattern evident among major gene families previously implicated in developmental processes. For example, expression of Knotted gene family members increased during the induction period, whereas transcription factors from the Wuschel gene family were unaltered during shoot induction. Expression levels of genes involved in cell cycle regulation increased steadily on regeneration medium while expression of NAC genes varied. No obvious possible candidate genes or developmental processes could be identified as a target for the early events (first few days) in the induction of shoot regeneration. On the other hand, observations during the early stages of regeneration pointed out that regeneration does not occur from a single cell but a group of cells. We observed that while cell division starts just as leaf segments are placed on regeneration medium, only a group of cells could become shoot primordia. Still, these primordia are not identifiable during the first days.
Keywords: regeneration induction; shoot regeneration; tobacco.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Steward F.C. Growth and Organized Development of Cultured Cells. III. Interpretations of the Growth from Free Cell to Carrot Plant. Am. J. Bot. 1958;45:709–713. doi: 10.1002/j.1537-2197.1958.tb10600.x. - DOI
LinkOut - more resources
Full Text Sources

