Iron Dysregulation and Inflammagens Related to Oral and Gut Health Are Central to the Development of Parkinson's Disease
- PMID: 33383805
- PMCID: PMC7823713
- DOI: 10.3390/biom11010030
Iron Dysregulation and Inflammagens Related to Oral and Gut Health Are Central to the Development of Parkinson's Disease
Abstract
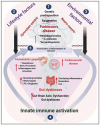
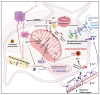
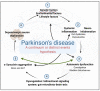
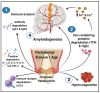
Neuronal lesions in Parkinson's disease (PD) are commonly associated with α-synuclein (α-Syn)-induced cell damage that are present both in the central and peripheral nervous systems of patients, with the enteric nervous system also being especially vulnerable. Here, we bring together evidence that the development and presence of PD depends on specific sets of interlinking factors that include neuroinflammation, systemic inflammation, α-Syn-induced cell damage, vascular dysfunction, iron dysregulation, and gut and periodontal dysbiosis. We argue that there is significant evidence that bacterial inflammagens fuel this systemic inflammation, and might be central to the development of PD. We also discuss the processes whereby bacterial inflammagens may be involved in causing nucleation of proteins, including of α-Syn. Lastly, we review evidence that iron chelation, pre-and probiotics, as well as antibiotics and faecal transplant treatment might be valuable treatments in PD. A most important consideration, however, is that these therapeutic options need to be validated and tested in randomized controlled clinical trials. However, targeting underlying mechanisms of PD, including gut dysbiosis and iron toxicity, have potentially opened up possibilities of a wide variety of novel treatments, which may relieve the characteristic motor and nonmotor deficits of PD, and may even slow the progression and/or accompanying gut-related conditions of the disease.
Keywords: Parkinson’s disease; amyloid and α-synuclein; bacteria; gingipains; iron; lipopolysaccharides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The link between the gut microbiota and Parkinson's Disease: A systematic mechanism review with focus on α-synuclein transport.Brain Res. 2021 Oct 15;1769:147609. doi: 10.1016/j.brainres.2021.147609. Epub 2021 Aug 8. Brain Res. 2021. PMID: 34371014
-
Implications of the Gut Microbiome in Parkinson's Disease.Mov Disord. 2020 Jun;35(6):921-933. doi: 10.1002/mds.28004. Epub 2020 Feb 24. Mov Disord. 2020. PMID: 32092186 Review.
-
Is Gut Dysbiosis an Epicenter of Parkinson's Disease?Neurochem Res. 2021 Mar;46(3):425-438. doi: 10.1007/s11064-020-03187-9. Epub 2021 Jan 5. Neurochem Res. 2021. PMID: 33400024 Review.
-
Human gut microbiota and Parkinson's disease.Prog Mol Biol Transl Sci. 2022;192(1):281-307. doi: 10.1016/bs.pmbts.2022.08.004. Epub 2022 Oct 3. Prog Mol Biol Transl Sci. 2022. PMID: 36280322 Review.
-
Oral and intestinal dysbiosis in Parkinson's disease.Rev Neurol (Paris). 2023 Nov;179(9):937-946. doi: 10.1016/j.neurol.2022.12.010. Epub 2023 Mar 16. Rev Neurol (Paris). 2023. PMID: 36934020 Review.
Cited by
-
Revisiting Alpha-Synuclein Pathways to Inflammation.Int J Mol Sci. 2023 Apr 12;24(8):7137. doi: 10.3390/ijms24087137. Int J Mol Sci. 2023. PMID: 37108299 Free PMC article. Review.
-
Bioactive strawberry fruit (Arbutus unedo L.) extract remedies paraquat-induced neurotoxicity in the offspring prenatally exposed rats.Front Neurosci. 2023 Oct 12;17:1244603. doi: 10.3389/fnins.2023.1244603. eCollection 2023. Front Neurosci. 2023. PMID: 37901424 Free PMC article.
-
A mitochondrial inside-out iron-calcium signal reveals drug targets for Parkinson's disease.Cell Rep. 2023 Dec 26;42(12):113544. doi: 10.1016/j.celrep.2023.113544. Epub 2023 Dec 6. Cell Rep. 2023. PMID: 38060381 Free PMC article.
-
Blood-Brain Barrier Disruption by Lipopolysaccharide and Sepsis-Associated Encephalopathy.Front Cell Infect Microbiol. 2021 Nov 4;11:768108. doi: 10.3389/fcimb.2021.768108. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34804998 Free PMC article. Review.
-
A Direct Relationship Between 'Blood Stasis' and Fibrinaloid Microclots in Chronic, Inflammatory, and Vascular Diseases, and Some Traditional Natural Products Approaches to Treatment.Pharmaceuticals (Basel). 2025 May 12;18(5):712. doi: 10.3390/ph18050712. Pharmaceuticals (Basel). 2025. PMID: 40430532 Free PMC article. Review.
References
-
- Dorsey E.R., Elbaz A., Nichols E., Abd-Allah F., Abdelalim A., Adsuar J.C., Ansha M.G., Brayne C., Choi J.-Y.J., Collado-Mateo D. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

