Broadly Active Antiviral Compounds Disturb Zika Virus Progeny Release Rescuing Virus-Induced Toxicity in Brain Organoids
- PMID: 33383826
- PMCID: PMC7823652
- DOI: 10.3390/v13010037
Broadly Active Antiviral Compounds Disturb Zika Virus Progeny Release Rescuing Virus-Induced Toxicity in Brain Organoids
Abstract
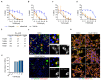
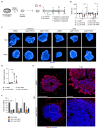
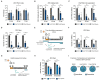
RNA viruses have gained plenty of attention during recent outbreaks of Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Zika virus (ZIKV), and Ebola virus. ZIKV is a vector borne Flavivirus that is spread by mosquitoes and it mainly infects neuronal progenitor cells. One hallmark of congenital ZIKV disease is a reduced brain size in fetuses, leading to severe neurological defects. The World Health Organization (WHO) is urging the development of new antiviral treatments against ZIKV, as there are no efficient countermeasures against ZIKV disease. Previously, we presented a new class of host-targeting antivirals active against a number of pathogenic RNA viruses, such as SARS-CoV-2. Here, we show the transfer of the image-based phenotypic antiviral assay to ZIKV-infected brain cells, followed by mechanism-of-action studies and a proof-of-concept study in a three-dimensional (3D) organoid model. The novel antiviral compounds showed a therapeutic window against ZIKV in several cell models and rescued ZIKV-induced neurotoxicity in brain organoids. The compound's mechanism-of-action was pinpointed to late steps in the virus life cycle, impairing the formation of new virus particles. Collectively, in this study, we expand the antiviral activity of new small molecule inhibitors to a new virus class of Flaviviruses, but also uncover compounds' mechanism of action, which are important for the further development of antivirals.
Keywords: Zika virus; antivirals; brain organoids; mode-of-action; pathogenic RNA viruses.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- WHO Prioritizing Diseases for Research and Development in Emergency Contexts. [(accessed on 26 November 2020)]; Available online: https://www.who.int/activities/prioritizing-diseases-for-research-and-de....
-
- Souza B.S.F., Sampaio G.L.A., Pereira C.S., Campos G.S., Sardi S.I., Freitas L.A.R., Figueira C.P., Paredes B.D., Nonaka C.K.V., Azevedo C.M., et al. Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells. Sci. Rep. 2016 doi: 10.1038/srep39775. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

