The Role of Hypoxia-Inducible Factor Post-Translational Modifications in Regulating Its Localisation, Stability, and Activity
- PMID: 33383924
- PMCID: PMC7796330
- DOI: 10.3390/ijms22010268
The Role of Hypoxia-Inducible Factor Post-Translational Modifications in Regulating Its Localisation, Stability, and Activity
Abstract
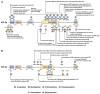
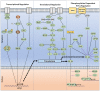
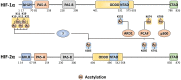
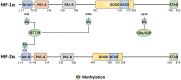
The hypoxia signalling pathway enables adaptation of cells to decreased oxygen availability. When oxygen becomes limiting, the central transcription factors of the pathway, hypoxia-inducible factors (HIFs), are stabilised and activated to induce the expression of hypoxia-regulated genes, thereby maintaining cellular homeostasis. Whilst hydroxylation has been thoroughly described as the major and canonical modification of the HIF-α subunits, regulating both HIF stability and activity, a range of other post-translational modifications decorating the entire protein play also a crucial role in altering HIF localisation, stability, and activity. These modifications, their conservation throughout evolution, and their effects on HIF-dependent signalling are discussed in this review.
Keywords: HIF-1α; HIF-2α; S-nitrosylation; acetylation; cysteine phosphorylation; hypoxia; methylation; phosphorylation; posttranslational modifications; signalling; sumoylation; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ema M., Taya S., Yokotani N., Sogawa K., Matsuda Y., Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl. Acad. Sci. USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

