Involvement of the Spinal Cord in Primary Mitochondrial Disorders: A Neuroimaging Mimicker of Inflammation and Ischemia in Children
- PMID: 33384291
- PMCID: PMC7872189
- DOI: 10.3174/ajnr.A6910
Involvement of the Spinal Cord in Primary Mitochondrial Disorders: A Neuroimaging Mimicker of Inflammation and Ischemia in Children
Abstract
Background and purpose: Little is known about imaging features of spinal cord lesions in mitochondrial disorders. The aim of this research was to assess the frequency, imaging features, and pathogenic variants causing primary mitochondrial disease in children with spinal cord lesions.
Materials and methods: This retrospective analysis included patients seen at Children's Hospital of Philadelphia between 2000 and 2019 who had a confirmed diagnosis of a primary (genetic-based) mitochondrial disease and available MR imaging of the spine. The MR imaging included at least both sagittal and axial fast spin-echo T2-weighted images. Spine images were independently reviewed by 2 neuroradiologists. Location and imaging features of spinal cord lesions were correlated and tested using the Fisher exact test.
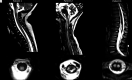
Results: Of 119 children with primary mitochondrial disease in whom MR imaging was available, only 33 of 119 (28%) had available spine imaging for reanalysis. Nineteen of these 33 individuals (58%) had evidence of spinal cord lesions. Two main patterns of spinal cord lesions were identified: group A (12/19; 63%) had white ± gray matter involvement, and group B (7/19; 37%) had isolated gray matter involvement. Group A spinal cord lesions were similar to those seen in patients with neuromyelitis optica spectrum disorder, multiple sclerosis, anti-myelin oligodendrocyte glycoprotein-IgG antibody disease, and leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Group B patients had spinal cord findings similar to those that occur with ischemia and viral infections. Significant associations were seen between the pattern of lesions (group A versus group B) and the location of lesions in cervical versus thoracolumbar segments, respectively (P < .01).
Conclusions: Spinal cord lesions are frequently observed in children with primary mitochondrial disease and may mimic more common causes such as demyelination and ischemia.
© 2021 by American Journal of Neuroradiology.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
