Engineering exosome polymer hybrids by atom transfer radical polymerization
- PMID: 33384328
- PMCID: PMC7812758
- DOI: 10.1073/pnas.2020241118
Engineering exosome polymer hybrids by atom transfer radical polymerization
Abstract
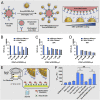
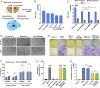
Exosomes are emerging as ideal drug delivery vehicles due to their biological origin and ability to transfer cargo between cells. However, rapid clearance of exogenous exosomes from the circulation as well as aggregation of exosomes and shedding of surface proteins during storage limit their clinical translation. Here, we demonstrate highly controlled and reversible functionalization of exosome surfaces with well-defined polymers that modulate the exosome's physiochemical and pharmacokinetic properties. Using cholesterol-modified DNA tethers and complementary DNA block copolymers, exosome surfaces were engineered with different biocompatible polymers. Additionally, polymers were directly grafted from the exosome surface using biocompatible photo-mediated atom transfer radical polymerization (ATRP). These exosome polymer hybrids (EPHs) exhibited enhanced stability under various storage conditions and in the presence of proteolytic enzymes. Tuning of the polymer length and surface loading allowed precise control over exosome surface interactions, cellular uptake, and preserved bioactivity. EPHs show fourfold higher blood circulation time without altering tissue distribution profiles. Our results highlight the potential of precise nanoengineering of exosomes toward developing advanced drug and therapeutic delivery systems using modern ATRP methods.
Keywords: ATRP; exosome; polymer; polymer biohybrid.
Conflict of interest statement
The authors declare no competing interest.
Figures




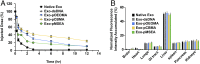
Similar articles
-
Controlled Release of Exosomes Using Atom Transfer Radical Polymerization-Based Hydrogels.Biomacromolecules. 2022 Apr 11;23(4):1713-1722. doi: 10.1021/acs.biomac.1c01636. Epub 2022 Mar 18. Biomacromolecules. 2022. PMID: 35302760
-
Nanogel hybrid assembly for exosome intracellular delivery: effects on endocytosis and fusion by exosome surface polymer engineering.Biomater Sci. 2020 Jan 21;8(2):619-630. doi: 10.1039/c9bm01232j. Biomater Sci. 2020. PMID: 31833484
-
PNIPAM grafted surfaces through ATRP and RAFT polymerization: Chemistry and bioadhesion.Colloids Surf B Biointerfaces. 2017 Mar 1;151:143-155. doi: 10.1016/j.colsurfb.2016.12.007. Epub 2016 Dec 7. Colloids Surf B Biointerfaces. 2017. PMID: 27992845 Review.
-
Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications.Int J Nanomedicine. 2023 Dec 23;18:7923-7940. doi: 10.2147/IJN.S444582. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38152837 Free PMC article. Review.
-
The Effect of Covalently-Attached ATRP-Synthesized Polymers on Membrane Stability and Cytoprotection in Human Erythrocytes.PLoS One. 2016 Jun 22;11(6):e0157641. doi: 10.1371/journal.pone.0157641. eCollection 2016. PLoS One. 2016. PMID: 27331401 Free PMC article.
Cited by
-
Exploring the role of polymers to overcome ongoing challenges in the field of extracellular vesicles.J Extracell Vesicles. 2023 Dec;12(12):e12386. doi: 10.1002/jev2.12386. J Extracell Vesicles. 2023. PMID: 38050832 Free PMC article. Review.
-
Highly Sensitive Detection of Bacteria by Binder-Coupled Multifunctional Polymeric Dyes.Polymers (Basel). 2023 Jun 18;15(12):2723. doi: 10.3390/polym15122723. Polymers (Basel). 2023. PMID: 37376368 Free PMC article.
-
Open-air green-light-driven ATRP enabled by dual photoredox/copper catalysis.Chem Sci. 2022 Sep 20;13(39):11540-11550. doi: 10.1039/d2sc04210j. eCollection 2022 Oct 12. Chem Sci. 2022. PMID: 36320395 Free PMC article.
-
Unlocking the promise of mRNA therapeutics.Nat Biotechnol. 2022 Nov;40(11):1586-1600. doi: 10.1038/s41587-022-01491-z. Epub 2022 Nov 3. Nat Biotechnol. 2022. PMID: 36329321 Review.
-
Gut Microbiota-Derived Small Extracellular Vesicles Endorse Memory-like Inflammatory Responses in Murine Neutrophils.Biomedicines. 2022 Feb 14;10(2):442. doi: 10.3390/biomedicines10020442. Biomedicines. 2022. PMID: 35203650 Free PMC article.
References
-
- Kourembanas S., Exosomes: Vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu. Rev. Physiol. 77, 13–27 (2015). - PubMed
-
- Colombo M., Raposo G., Théry C., Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

