A chemiresistive methane sensor
- PMID: 33384329
- PMCID: PMC7812817
- DOI: 10.1073/pnas.2022515118
A chemiresistive methane sensor
Abstract
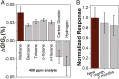
A chemiresistive sensor is described for the detection of methane (CH4), a potent greenhouse gas that also poses an explosion hazard in air. The chemiresistor allows for the low-power, low-cost, and distributed sensing of CH4 at room temperature in air with environmental implications for gas leak detection in homes, production facilities, and pipelines. Specifically, the chemiresistors are based on single-walled carbon nanotubes (SWCNTs) noncovalently functionalized with poly(4-vinylpyridine) (P4VP) that enables the incorporation of a platinum-polyoxometalate (Pt-POM) CH4 oxidation precatalyst into the sensor by P4VP coordination. The resulting SWCNT-P4VP-Pt-POM composite showed ppm-level sensitivity to CH4 and good stability to air as well as time, wherein the generation of a high-valent platinum intermediate during CH4 oxidation is proposed as the origin of the observed chemiresistive response. The chemiresistor was found to exhibit selectivity for CH4 over heavier hydrocarbons such as n-hexane, benzene, toluene, and o-xylene, as well as gases, including carbon dioxide and hydrogen. The utility of the sensor in detecting CH4 using a simple handheld multimeter was also demonstrated.
Keywords: catalysis; chemiresistors; methane; selectors; sensor.
Conflict of interest statement
Competing interest statement: A patent has been filed on this technology.
Figures




Similar articles
-
Application of Semiconductor Metal Oxide in Chemiresistive Methane Gas Sensor: Recent Developments and Future Perspectives.Molecules. 2023 Sep 20;28(18):6710. doi: 10.3390/molecules28186710. Molecules. 2023. PMID: 37764486 Free PMC article. Review.
-
Continuous Flow Chemistry and Bayesian Optimization for Polymer-Functionalized Carbon Nanotube-Based Chemiresistive Methane Sensors.ACS Appl Mater Interfaces. 2024 Dec 11;16(49):68181-68196. doi: 10.1021/acsami.4c14279. Epub 2024 Nov 26. ACS Appl Mater Interfaces. 2024. PMID: 39592136 Free PMC article.
-
Trace Hydrogen Sulfide Sensing Inspired by Polyoxometalate-Mediated Aerobic Oxidation.ACS Cent Sci. 2021 Sep 22;7(9):1572-1580. doi: 10.1021/acscentsci.1c00746. Epub 2021 Aug 30. ACS Cent Sci. 2021. PMID: 34584959 Free PMC article.
-
Quaternized Polymer-Single-Walled Carbon Nanotube Scaffolds for a Chemiresistive Glucose Sensor.ACS Sens. 2017 Aug 25;2(8):1123-1127. doi: 10.1021/acssensors.7b00323. Epub 2017 Jul 31. ACS Sens. 2017. PMID: 28758726
-
Designing oxide chemiresistors for detecting volatile aromatic compounds: recent progresses and future perspectives.Chem Commun (Camb). 2022 May 3;58(36):5439-5454. doi: 10.1039/d2cc01563c. Chem Commun (Camb). 2022. PMID: 35415739 Review.
Cited by
-
Research Progress on Chemiresistive Carbon Monoxide Sensors.Nanomaterials (Basel). 2025 Feb 16;15(4):303. doi: 10.3390/nano15040303. Nanomaterials (Basel). 2025. PMID: 39997866 Free PMC article. Review.
-
Application of Semiconductor Metal Oxide in Chemiresistive Methane Gas Sensor: Recent Developments and Future Perspectives.Molecules. 2023 Sep 20;28(18):6710. doi: 10.3390/molecules28186710. Molecules. 2023. PMID: 37764486 Free PMC article. Review.
-
Gas nanosensors for health and safety applications in mining.Nanoscale Adv. 2023 Oct 24;5(22):5997-6016. doi: 10.1039/d3na00507k. eCollection 2023 Nov 7. Nanoscale Adv. 2023. PMID: 37941945 Free PMC article. Review.
-
The Progress of Research into Flexible Sensors in the Field of Smart Wearables.Sensors (Basel). 2022 Jul 6;22(14):5089. doi: 10.3390/s22145089. Sensors (Basel). 2022. PMID: 35890768 Free PMC article. Review.
-
Continuous Flow Chemistry and Bayesian Optimization for Polymer-Functionalized Carbon Nanotube-Based Chemiresistive Methane Sensors.ACS Appl Mater Interfaces. 2024 Dec 11;16(49):68181-68196. doi: 10.1021/acsami.4c14279. Epub 2024 Nov 26. ACS Appl Mater Interfaces. 2024. PMID: 39592136 Free PMC article.
References
-
- Myhre G., et al. , "Anthropogenic and natural radiative forcing” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Stocker T. F. et al., Eds. (Cambridge University Press, Cambridge, UK, 2013), pp. 659–740.
-
- Zabetakis M. G., Flammability characteristics of combustible gases and vapors. U.S. Bureau of Mines Bulletin 627, 1–121 (1965).
-
- Dlugokencky E. J., Steele L. P., Lang P. M., Masarie K. A., The growth rate and distribution of atmospheric methane. J. Geophys. Res. 99, 17021–17043 (1994).
-
- Shemshad J., Aminossadati S. M., Kizil M. S., A review of developments in near infrared methane detection based on tunable diode laser. Sens. Actuators B Chem. 171-172, 77–92 (2012).
Publication types
LinkOut - more resources
Full Text Sources

