First-line treatment with infliximab versus conventional treatment in children with newly diagnosed moderate-to-severe Crohn's disease: an open-label multicentre randomised controlled trial
- PMID: 33384335
- PMCID: PMC8666701
- DOI: 10.1136/gutjnl-2020-322339
First-line treatment with infliximab versus conventional treatment in children with newly diagnosed moderate-to-severe Crohn's disease: an open-label multicentre randomised controlled trial
Abstract
Objective: In newly diagnosed paediatric patients with moderate-to-severe Crohn's disease (CD), infliximab (IFX) is initiated once exclusive enteral nutrition (EEN), corticosteroid and immunomodulator therapies have failed. We aimed to investigate whether starting first-line IFX (FL-IFX) is more effective to achieve and maintain remission than conventional treatment.
Design: In this multicentre open-label randomised controlled trial, untreated patients with a new diagnosis of CD (3-17 years old, weighted Paediatric CD Activity Index score (wPCDAI) >40) were assigned to groups that received five infusions of 5 mg/kg IFX at weeks 0, 2, 6, 14 and 22 (FL-IFX), or EEN or oral prednisolone (1 mg/kg, maximum 40 mg) (conventional). The primary outcome was clinical remission on azathioprine, defined as a wPCDAI <12.5 at week 52, without need for treatment escalation, using intention-to-treat analysis.
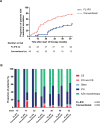
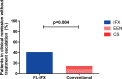
Results: 100 patients were included, 50 in the FL-IFX group and 50 in the conventional group. Four patients did not receive treatment as per protocol. At week 10, a higher proportion of patients in the FL-IFX group than in the conventional group achieved clinical (59% vs 34%, respectively, p=0.021) and endoscopic remission (59% vs 17%, respectively, p=0.001). At week 52, the proportion of patients in clinical remission was not significantly different (p=0.421). However, 19/46 (41%) patients in the FL-IFX group were in clinical remission on azathioprine monotherapy without need for treatment escalation vs 7/48 (15%) in the conventional group (p=0.004).
Conclusions: FL-IFX was superior to conventional treatment in achieving short-term clinical and endoscopic remission, and had greater likelihood of maintaining clinical remission at week 52 on azathioprine monotherapy.
Trial registration number: ClinicalTrials.gov Registry (NCT02517684).
Keywords: IBD clinical; inflammatory bowel disease; infliximab; paediatric gastroenterology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: LdR reports grants from ZonMW, ECCO, Crocokids and Pfizer and consultancy fees from Abbvie, during the conduct of the study. MAA received a consultant fee from Abbvie, outside the submitted work. MAC reports grants from ZonMw and Crocokids, and grants and non-financial support from Pfizer during the conduct of the study. IH received a payment/honorarium for lectures from BioGaia, Nutricia, Oktal pharma, Nestle, Biocodex and AbelaPharm. K-LK received consultant fees from Abbvie, Biocodex, Ferring, MSD and Tillotts Pharma, and research grants from the Pediatric Research Foundation (Finland) and the Helsinki University Research Fund, outside the submitted work. TH received a consultant fee from Pfizer, outside the submitted work. JS reports personal fees from Nutricia, outside the submitted work. MPvW reports personal fees from Danone and Laborie, outside the submitted work. STAT-R received a consultant fee from Pfizer, outside the submitted work. JCE received consultant fees from Abbvie and Janssen, as well as research support from MSD and Nutricia.
Figures

Comment in
-
Infliximab at diagnosis: moving towards personalisation in paediatric inflammatory bowel disease.Gut. 2022 Jan;71(1):2-3. doi: 10.1136/gutjnl-2021-324214. Epub 2021 Mar 15. Gut. 2022. PMID: 33722861 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
