Using biochemistry and biophysics to extinguish androgen receptor signaling in prostate cancer
- PMID: 33384381
- PMCID: PMC7949100
- DOI: 10.1074/jbc.REV120.012411
Using biochemistry and biophysics to extinguish androgen receptor signaling in prostate cancer
Abstract
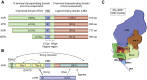
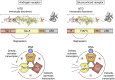
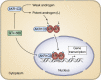
Castration resistant prostate cancer (CRPC) continues to be androgen receptor (AR) driven. Inhibition of AR signaling in CRPC could be advanced using state-of-the-art biophysical and biochemical techniques. Structural characterization of AR and its complexes by cryo-electron microscopy would advance the development of N-terminal domain (NTD) and ligand-binding domain (LBD) antagonists. The structural basis of AR function is unlikely to be determined by any single structure due to the intrinsic disorder of its NTD, which not only interacts with coregulators but likely accounts for the constitutive activity of AR-splice variants (SV), which lack the LBD and emerge in CRPC. Using different AR constructs lacking the LBD, their effects on protein folding, DNA binding, and transcriptional activity could reveal how interdomain coupling explains the activity of AR-SVs. The AR also interacts with coregulators that promote chromatin looping. Elucidating the mechanisms involved can identify vulnerabilities to treat CRPC, which do not involve targeting the AR. Phosphorylation of the AR coactivator MED-1 by CDK7 is one mechanism that can be blocked by the use of CDK7 inhibitors. CRPC gains resistance to AR signaling inhibitors (ARSI). Drug resistance may involve AR-SVs, but their role requires their reliable quantification by SILAC-mass spectrometry during disease progression. ARSI drug resistance also occurs by intratumoral androgen biosynthesis catalyzed by AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase), which is unique in that its acts as a coactivator of AR. Novel bifunctional inhibitors that competitively inhibit AKR1C3 and block its coactivator function could be developed using reverse-micelle NMR and fragment-based drug discovery.
Keywords: aldo-keto reductase; allostery; androgen receptor; cryo-electron microscopy; mass spectrometry; nuclear magnetic resonance; proteomics; splice variants.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest T. M. P. is the founder of Penzymes, LLC; he receives sponsored research funding from Forendo and serves on the Expert Panel for the Research Institute for Fragrance Materials. I. A. B is a founder of Proteoform Bio and a paid consultant for Calico, Chimerix, PTC Therapeutics, Takeda Pharmaceuticals, and Vivo Capital. S. R. P is the president of ProsTech, Inc. All other authors declare no competing interests.
Figures



References
-
- Siegel R.L., Miller K.D., Jermal A. Cancer statistics. CA Cancer J. Clin. 2019;69:7–34. - PubMed
-
- Sharifi R., Soloway M. Clinical study of leuprolide depot formulation in the treatment of advanced prostate cancer. The Leuprolide Study Group. J. Urol. 1990;143:68–71. - PubMed
-
- Querol Cano L., Lavery D.N., Bevan C.L. Mini-review: Foldosome regulation of androgen receptor action in prostate cancer. Mol. Cell. Endocrinol. 2013;369:52–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

