O-acetylation controls the glycosylation of bacterial serine-rich repeat glycoproteins
- PMID: 33384382
- PMCID: PMC7948813
- DOI: 10.1074/jbc.RA120.016116
O-acetylation controls the glycosylation of bacterial serine-rich repeat glycoproteins
Abstract
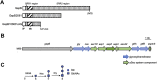
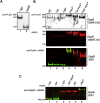
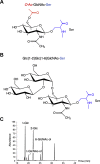
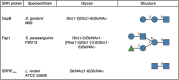
The serine-rich repeat (SRR) glycoproteins of gram-positive bacteria are a family of adhesins that bind to a wide range of host ligands, and expression of SRR glycoproteins is linked with enhanced bacterial virulence. The biogenesis of these surface glycoproteins involves their intracellular glycosylation and export via the accessory Sec system. Although all accessory Sec components are required for SRR glycoprotein export, Asp2 of Streptococcus gordonii also functions as an O-acetyltransferase that modifies GlcNAc residues on the SRR adhesin gordonii surface protein B (GspB). Because these GlcNAc residues can also be modified by the glycosyltransferases Nss and Gly, it has been unclear whether the post-translational modification of GspB is coordinated. We now report that acetylation modulates the glycosylation of exported GspB. Loss of O-acetylation due to aps2 mutagenesis led to the export of GspB glycoforms with increased glucosylation of the GlcNAc moieties. Linkage analysis of the GspB glycan revealed that both O-acetylation and glucosylation occurred at the same C6 position on GlcNAc residues and that O-acetylation prevented Glc deposition. Whereas streptococci expressing nonacetylated GspB with increased glucosylation were significantly reduced in their ability to bind human platelets in vitro, deletion of the glycosyltransferases nss and gly in the asp2 mutant restored platelet binding to WT levels. These findings demonstrate that GlcNAc O-acetylation controls GspB glycosylation, such that binding via this adhesin is optimized. Moreover, because O-acetylation has comparable effects on the glycosylation of other SRR adhesins, acetylation may represent a conserved regulatory mechanism for the post-translational modification of the SRR glycoprotein family.
Keywords: O-acetylation; Streptococcus gordonii; accessory; glycoprotein:glucosylation.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Rose L., Shivshankar P., Hinojosa E., Rodriguez A., Sanchez C.J., Orihuela C.J. Antibodies against PsrP, a novel Streptococcus pneumoniae adhesin, block adhesion and protect mice against pneumococcal challenge. J. Infect. Dis. 2008;198:375–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

