Magnetic spatiotemporal control of SOS1 coupled nanoparticles for guided neurite growth in dopaminergic single cells
- PMID: 33384447
- PMCID: PMC7775457
- DOI: 10.1038/s41598-020-80253-w
Magnetic spatiotemporal control of SOS1 coupled nanoparticles for guided neurite growth in dopaminergic single cells
Abstract
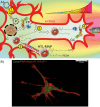
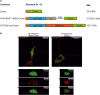
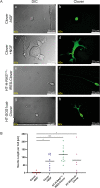
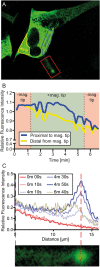
The axon regeneration of neurons in the brain can be enhanced by activating intracellular signaling pathways such as those triggered by the membrane-anchored Rat sarcoma (RAS) proto-oncogene. Here we demonstrate the induction of neurite growth by expressing tagged permanently active Harvey-RAS protein or the RAS-activating catalytic domain of the guanine nucleotide exchange factor (SOS1cat), in secondary dopaminergic cells. Due to the tag, the expressed fusion protein is captured by functionalized magnetic nanoparticles in the cytoplasm of the cell. We use magnetic tips for remote translocation of the SOS1cat-loaded magnetic nanoparticles from the cytoplasm towards the inner face of the plasma membrane where the endogenous Harvey-RAS protein is located. Furthermore, we show the magnetic transport of SOS1cat-bound nanoparticles from the cytoplasm into the neurite until they accumulate at its tip on a time scale of minutes. In order to scale-up from single cells, we show the cytoplasmic delivery of the magnetic nanoparticles into large numbers of cells without changing the cellular response to nerve growth factor. These results will serve as an initial step to develop tools for refining cell replacement therapies based on grafted human induced dopaminergic neurons loaded with functionalized magnetic nanoparticles in Parkinson model systems.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Novel Tools towards Magnetic Guidance of Neurite Growth: (I) Guidance of Magnetic Nanoparticles into Neurite Extensions of Induced Human Neurons and In Vitro Functionalization with RAS Regulating Proteins.J Funct Biomater. 2019 Jul 16;10(3):32. doi: 10.3390/jfb10030032. J Funct Biomater. 2019. PMID: 31315182 Free PMC article.
-
Membrane-targeting potentiates guanine nucleotide exchange factor CDC25 and SOS1 activation of Ras transforming activity.Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8512-6. doi: 10.1073/pnas.91.18.8512. Proc Natl Acad Sci U S A. 1994. PMID: 8078913 Free PMC article.
-
Direct interaction of SOS1 Ras exchange protein with the SH3 domain of phospholipase C-gamma1.Biochemistry. 2000 Jul 25;39(29):8674-82. doi: 10.1021/bi992558t. Biochemistry. 2000. PMID: 10913276
-
Control of ras activation.Cancer Surv. 1996;27:87-100. Cancer Surv. 1996. PMID: 8909796 Review.
-
Noonan, Costello and cardio-facio-cutaneous syndromes: dysregulation of the Ras-MAPK pathway.Expert Rev Mol Med. 2008 Dec 9;10:e37. doi: 10.1017/S1462399408000902. Expert Rev Mol Med. 2008. PMID: 19063751 Review.
Cited by
-
Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering.Nanomaterials (Basel). 2021 Sep 8;11(9):2337. doi: 10.3390/nano11092337. Nanomaterials (Basel). 2021. PMID: 34578651 Free PMC article. Review.
-
Directional control of neurite outgrowth: emerging technologies for Parkinson's disease using magnetic nanoparticles and magnetic field gradients.J R Soc Interface. 2022 Nov;19(196):20220576. doi: 10.1098/rsif.2022.0576. Epub 2022 Nov 9. J R Soc Interface. 2022. PMID: 36349444 Free PMC article.
-
A comprehensive approach to characterize navigation instruments for magnetic guidance in biological systems.Sci Rep. 2024 Apr 3;14(1):7879. doi: 10.1038/s41598-024-58091-x. Sci Rep. 2024. PMID: 38570608 Free PMC article.
-
Magnetogenetics: remote activation of cellular functions triggered by magnetic switches.Nanoscale. 2022 Feb 10;14(6):2091-2118. doi: 10.1039/d1nr06303k. Nanoscale. 2022. PMID: 35103278 Free PMC article. Review.
-
Intracellular Proteins Targeting with Bi-Functionalized Magnetic Nanoparticles Following their Endosomal Escape.Small. 2025 Apr;21(13):e2410454. doi: 10.1002/smll.202410454. Epub 2025 Feb 19. Small. 2025. PMID: 39967470 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

