Extracellular histones stimulate collagen expression in vitro and promote liver fibrogenesis in a mouse model via the TLR4-MyD88 signaling pathway
- PMID: 33384551
- PMCID: PMC7754552
- DOI: 10.3748/wjg.v26.i47.7513
Extracellular histones stimulate collagen expression in vitro and promote liver fibrogenesis in a mouse model via the TLR4-MyD88 signaling pathway
Abstract
Background: Liver fibrosis progressing to liver cirrhosis and hepatic carcinoma is very common and causes more than one million deaths annually. Fibrosis develops from recurrent liver injury but the molecular mechanisms are not fully understood. Recently, the TLR4-MyD88 signaling pathway has been reported to contribute to fibrosis. Extracellular histones are ligands of TLR4 but their roles in liver fibrosis have not been investigated.
Aim: To investigate the roles and potential mechanisms of extracellular histones in liver fibrosis.
Methods: In vitro, LX2 human hepatic stellate cells (HSCs) were treated with histones in the presence or absence of non-anticoagulant heparin (NAHP) for neutralizing histones or TLR4-blocking antibody. The resultant cellular expression of collagen I was detected using western blotting and immunofluorescent staining. In vivo, the CCl4-induced liver fibrosis model was generated in male 6-week-old ICR mice and in TLR4 or MyD88 knockout and parental mice. Circulating histones were detected and the effect of NAHP was evaluated.
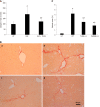
Results: Extracellular histones strongly stimulated LX2 cells to produce collagen I. Histone-enhanced collagen expression was significantly reduced by NAHP and TLR4-blocking antibody. In CCl4-treated wild type mice, circulating histones were dramatically increased and maintained high levels during the duration of fibrosis-induction. Injection of NAHP not only reduced alanine aminotransferase and liver injury scores, but also significantly reduced fibrogenesis. Since the TLR4-blocking antibody reduced histone-enhanced collagen I production in HSC, the CCl4 model with TLR4 and MyD88 knockout mice was used to demonstrate the roles of the TLR4-MyD88 signaling pathway in CCl4-induced liver fibrosis. The levels of liver fibrosis were indeed significantly reduced in knockout mice compared to wild type parental mice.
Conclusion: Extracellular histones potentially enhance fibrogenesis via the TLR4-MyD88 signaling pathway and NAHP has therapeutic potential by detoxifying extracellular histones.
Keywords: CCl4; Extracellular histones; Liver fibrosis; MyD88; Non-anticoagulant heparin; TLR4.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: No conflict interested has been claimed by any author.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

