Acetylcholine Muscarinic Receptors in Ventral Hippocampus Modulate Stress-Induced Anxiety-Like Behaviors in Mice
- PMID: 33384583
- PMCID: PMC7769836
- DOI: 10.3389/fnmol.2020.598811
Acetylcholine Muscarinic Receptors in Ventral Hippocampus Modulate Stress-Induced Anxiety-Like Behaviors in Mice
Abstract
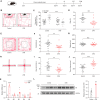
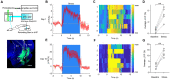
Chronic stress exposure increases the risk of developing various neuropsychiatric illnesses. The ventral hippocampus (vHPC) is central to affective and cognitive processing and displays a high density of acetylcholine (ACh) muscarinic receptors (mAChRs). However, the precise role of vHPC mAChRs in anxiety remains to be fully investigated. In this study, we found that chronic restraint stress (CRS) induced social avoidance and anxiety-like behaviors in mice and increased mAChR expression in the vHPC. CRS increased the vHPC ACh release in behaving mice. Moreover, CRS altered the synaptic activities and enhanced neuronal activity of the vHPC neurons. Using pharmacological and viral approaches, we showed that infusing the antagonist of mAChRs or decreasing their expression in the vHPC attenuated the anxiety-like behavior and rescued the social avoidance behaviors in mice probably due to suppression of vHPC neuronal activity and its excitatory synaptic transmission. Our results suggest that the changes of neuronal activity and synaptic transmission in the vHPC mediated by mAChRs may play an important role in stress-induced anxiety-like behavior, providing new insights into the pathological mechanism and potential pharmacological target for anxiety disorders.
Keywords: acetylcholine muscarinic receptors; anxiety; chronic stress; electrophysiology; hippocampus; scopolamine.
Copyright © 2020 Mei, Zhou, Sun, Liu, Zhang, Liu and Shu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bannerman D. M., Yee B. K., Good M. A., Heupel M. J., Iversen S. D., Rawlins J. N. P. (1999). Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav. Neurosci. 113, 1170–1188. 10.1037/0735-7044.113.6.1170 - DOI - PubMed
LinkOut - more resources
Full Text Sources

