The Roles of High Mobility Group Box 1 in Cerebral Ischemic Injury
- PMID: 33384585
- PMCID: PMC7770223
- DOI: 10.3389/fncel.2020.600280
The Roles of High Mobility Group Box 1 in Cerebral Ischemic Injury
Abstract
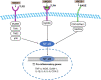
High mobility group box 1 (HMGB1) is a ubiquitous nuclear protein that plays an important role in stabilizing nucleosomes and DNA repair. HMGB1 can be passively released from necrotic neurons or actively secreted by microglia, macrophages/monocytes, and neutrophils. Cerebral ischemia is a major cause of mortality and disability worldwide, and its outcome depends on the number of neurons dying due to hypoxia in the ischemic area. HMGB1 contributes to the pathogenesis of cerebral ischemia via mediating neuroinflammatory responses to cerebral ischemic injury. Extracellular HMGB1 regulates many neuroinflammatory events by interacting with its different cell surface receptors, such as receptors for advanced glycation end products, toll-like receptor (TLR)-2, and TLR-4. Additionally, HMGB1 can be redox-modified, thus exerting specific cellular functions in the ischemic brain and has different roles in the acute and late stages of cerebral ischemic injury. However, the role of HMGB1 in cerebral ischemia is complex and remains unclear. Herein, we summarize and review the research on HMGB1 in cerebral ischemia, focusing especially on the role of HMGB1 in hypoxic ischemia in the immature brain and in white matter ischemic injury. We also outline the possible mechanisms of HMGB1 in cerebral ischemia and the main strategies to inhibit HMGB1 pertaining to its potential as a novel critical molecular target in cerebral ischemic injury.
Keywords: cerebral; high-mobility group box 1; ischemia; receptor for advanced glycation end products; therapeutic strategy; toll-like receptor.
Copyright © 2020 Gou, Ying, Yue, Qiu, Hu, Qu, Li and Mu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

