Salvianolic Acid B Protects Against Fatty Acid-Induced Renal Tubular Injury via Inhibition of Endoplasmic Reticulum Stress
- PMID: 33384598
- PMCID: PMC7770132
- DOI: 10.3389/fphar.2020.574229
Salvianolic Acid B Protects Against Fatty Acid-Induced Renal Tubular Injury via Inhibition of Endoplasmic Reticulum Stress
Abstract
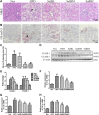
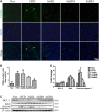
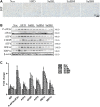
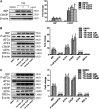
Background/Aims: Obesity-related kidney disease is associated with elevated levels of saturated free fatty acids (SFA). SFA lipotoxicity in tubular cells contributes to significant cellular apoptosis and injury. Salvianolic acid B (SalB) is the most abundant bioactive molecule from Radix Salviae Miltiorrhizae. In this study, we investigated the effect of SalB on SFA-induced renal tubular injury and endoplasmic reticulum (ER) stress, in vivo and in vitro. Methods: C57BL/6 mice were assigned to five groups: a control group with normal diet (Nor), high-fat diet group (HFD), and HFD with three different SalB treatment doses, low (SalBL; 3 mg/kg), medium (SalBM; 6.25 mg/kg), and high (SalBH; 12.5 mg/kg) doses. SalB was intraperitoneally injected daily for 4 weeks after 8 weeks of HFD. After 12 weeks, mice were sacrificed and kidneys and sera were collected. Apoptosis and ER stress were induced in human proximal tubule epitelial (HK2) cells by palmitic acid (PA, 0.6 mM), tunicamycin (TM, 1 μg/ml), or thapsigargin (TG, 200 nM) in vitro. Results: C57BL/6 mice fed a high-fat diet (HFD) for 12 weeks exhibited increased apoptosis (Bax and cleaved caspase-3) and ER stress (BIP, P-eIF2α, ATF4, CHOP, ATF6, IRE1α, and XBP1s) markers expression in the kidney, compared with control mice, which were remarkably suppressed by SalB treatment. In vitro studies showed that PA (0.6 mM) induced apoptosis and ER stress in cultured HK2 cells. SalB treatment attenuated all the adverse effects of PA. However, SalB failed to inhibit TM or TG-induced ER stress in HK2 cells. Conclusion: The study indicated that SalB may play an important role in obesity-related kidney injury via mediating SFA-induced ER stress.
Keywords: ER stress; apoptosis; renal tubular injury; salvianolic acid B; saturated fatty acid.
Copyright © 2020 Mai, Yin, Chen and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Intrarenal renin-angiotensin system mediates fatty acid-induced ER stress in the kidney.Am J Physiol Renal Physiol. 2016 Mar 1;310(5):F351-63. doi: 10.1152/ajprenal.00223.2015. Epub 2015 Dec 16. Am J Physiol Renal Physiol. 2016. PMID: 26672616 Free PMC article.
-
Combination of Chymostatin and Aliskiren attenuates ER stress induced by lipid overload in kidney tubular cells.Lipids Health Dis. 2018 Jul 31;17(1):183. doi: 10.1186/s12944-018-0818-1. Lipids Health Dis. 2018. PMID: 30064425 Free PMC article.
-
Protective Effects of Glucagon-Like Peptide-1 Analog on Renal Tubular Injury in Mice on High-Fat Diet.Cell Physiol Biochem. 2017;41(3):1113-1124. doi: 10.1159/000464118. Epub 2017 Feb 28. Cell Physiol Biochem. 2017. PMID: 28245463
-
Curcumin protects against palmitic acid-induced apoptosis via the inhibition of endoplasmic reticulum stress in testicular Leydig cells.Reprod Biol Endocrinol. 2019 Aug 31;17(1):71. doi: 10.1186/s12958-019-0517-4. Reprod Biol Endocrinol. 2019. PMID: 31472681 Free PMC article.
-
Salvianolic acid B in fibrosis treatment: a comprehensive review.Front Pharmacol. 2024 Jul 30;15:1442181. doi: 10.3389/fphar.2024.1442181. eCollection 2024. Front Pharmacol. 2024. PMID: 39139645 Free PMC article. Review.
Cited by
-
Natural products derived from traditional Chinese medicines targeting ER stress for the treatment of kidney diseases.Ren Fail. 2024 Dec;46(2):2396446. doi: 10.1080/0886022X.2024.2396446. Epub 2024 Aug 27. Ren Fail. 2024. PMID: 39192602 Free PMC article. Review.
-
Remifentanil attenuates endoplasmic reticulum stress and inflammatory injury in LPS-induced damage in HK-2 cells.Ren Fail. 2022 Dec;44(1):1769-1779. doi: 10.1080/0886022X.2022.2134028. Ren Fail. 2022. PMID: 36263441 Free PMC article.
-
Salvianolic Acid B: A Review of Pharmacological Effects, Safety, Combination Therapy, New Dosage Forms, and Novel Drug Delivery Routes.Pharmaceutics. 2023 Aug 29;15(9):2235. doi: 10.3390/pharmaceutics15092235. Pharmaceutics. 2023. PMID: 37765204 Free PMC article. Review.
-
High-Fat Diet-Induced Renal Proximal Tubular Inflammatory Injury: Emerging Risk Factor of Chronic Kidney Disease.Front Physiol. 2021 Dec 7;12:786599. doi: 10.3389/fphys.2021.786599. eCollection 2021. Front Physiol. 2021. PMID: 34950058 Free PMC article. Review.
-
New Insights Into the Effects of Individual Chinese Herbal Medicines on Chronic Kidney Disease.Front Pharmacol. 2021 Nov 4;12:774414. doi: 10.3389/fphar.2021.774414. eCollection 2021. Front Pharmacol. 2021. PMID: 34803715 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

