Cannabidiol as a Therapeutic Target: Evidence of its Neuroprotective and Neuromodulatory Function in Parkinson's Disease
- PMID: 33384602
- PMCID: PMC7770114
- DOI: 10.3389/fphar.2020.595635
Cannabidiol as a Therapeutic Target: Evidence of its Neuroprotective and Neuromodulatory Function in Parkinson's Disease
Abstract
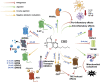
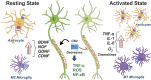
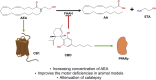
The phytocannabinoids of Cannabis sativa L. have, since ancient times, been proposed as a pharmacological alternative for treating various central nervous system (CNS) disorders. Interestingly, cannabinoid receptors (CBRs) are highly expressed in the basal ganglia (BG) circuit of both animals and humans. The BG are subcortical structures that regulate the initiation, execution, and orientation of movement. CBRs regulate dopaminergic transmission in the nigro-striatal pathway and, thus, the BG circuit also. The functioning of the BG is affected in pathologies related to movement disorders, especially those occurring in Parkinson's disease (PD), which produces motor and non-motor symptoms that involving GABAergic, glutamatergic, and dopaminergic neural networks. To date, the most effective medication for PD is levodopa (l-DOPA); however, long-term levodopa treatment causes a type of long-term dyskinesias, l-DOPA-induced dyskinesias (LIDs). With neuromodulation offering a novel treatment strategy for PD patients, research has focused on the endocannabinoid system (ECS), as it participates in the physiological neuromodulation of the BG in order to control movement. CBRs have been shown to inhibit neurotransmitter release, while endocannabinoids (eCBs) play a key role in the synaptic regulation of the BG. In the past decade, cannabidiol (CBD), a non-psychotropic phytocannabinoid, has been shown to have compensatory effects both on the ECS and as a neuromodulator and neuroprotector in models such as 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and reserpine, as well as other PD models. Although the CBD-induced neuroprotection observed in animal models of PD has been attributed to the activation of the CB1 receptor, recent research conducted at a molecular level has proposed that CBD is capable of activating other receptors, such as CB2 and the TRPV-1 receptor, both of which are expressed in the dopaminergic neurons of the nigro-striatal pathway. These findings open new lines of scientific inquiry into the effects of CBD at the level of neural communication. Cannabidiol activates the PPARγ, GPR55, GPR3, GPR6, GPR12, and GPR18 receptors, causing a variety of biochemical, molecular, and behavioral effects due to the broad range of receptors it activates in the CNS. Given the low number of pharmacological treatment alternatives for PD currently available, the search for molecules with the therapeutic potential to improve neuronal communication is crucial. Therefore, the investigation of CBD and the mechanisms involved in its function is required in order to ascertain whether receptor activation could be a treatment alternative for both PD and LID.
Keywords: cannabidiol (CBD); l-DOPA-induced dyskinesia; neuromodulatory; neuroprotective; parkinson’s diasese.
Copyright © 2020 Patricio, Morales-Andrade, Patricio-Martínez and Limón.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

