Biocontrol Traits Correlate With Resistance to Predation by Protists in Soil Pseudomonads
- PMID: 33384680
- PMCID: PMC7769776
- DOI: 10.3389/fmicb.2020.614194
Biocontrol Traits Correlate With Resistance to Predation by Protists in Soil Pseudomonads
Abstract
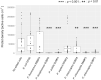
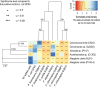
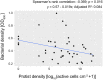
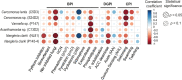
Root-colonizing bacteria can support plant growth and help fend off pathogens. It is clear that such bacteria benefit from plant-derived carbon, but it remains ambiguous why they invest in plant-beneficial traits. We suggest that selection via protist predation contributes to recruitment of plant-beneficial traits in rhizosphere bacteria. To this end, we examined the extent to which bacterial traits associated with pathogen inhibition coincide with resistance to protist predation. We investigated the resistance to predation of a collection of Pseudomonas spp. against a range of representative soil protists covering three eukaryotic supergroups. We then examined whether patterns of resistance to predation could be explained by functional traits related to plant growth promotion, disease suppression and root colonization success. We observed a strong correlation between resistance to predation and phytopathogen inhibition. In addition, our analysis highlighted an important contribution of lytic enzymes and motility traits to resist predation by protists. We conclude that the widespread occurrence of plant-protective traits in the rhizosphere microbiome may be driven by the evolutionary pressure for resistance against predation by protists. Protists may therefore act as microbiome regulators promoting native bacteria involved in plant protection against diseases.
Keywords: PGPR; biocontrol; multitrophic interactions; protozoa; rhizobacteria.
Copyright © 2020 Amacker, Gao, Agaras, Latz, Kowalchuk, Valverde, Jousset and Weidner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Agaras B. C., Noguera F., Anta G. G., Wall L., Valverde C. (2020). Biocontrol potential index of pseudomonads, instead of their direct-growth promotion traits, is a predictor of seed inoculation effect on crop productivity under field conditions. Biol. Control 143:104209 10.1016/j.biocontrol.2020.104209 - DOI
-
- Agaras B. C., Scandiani M., Luque A., Fernández L., Farina F., Carmona M., et al. (2015). Quantification of the potential biocontrol and direct plant growth promotion abilities based on multiple biological traits distinguish different groups of Pseudomonas Spp. Isolates. Biol. Control 90 173–186. 10.1016/j.biocontrol.2015.07.003 - DOI
-
- Andersen K. S., Winding A. (2004). Non-target effects of bacterial biological control agents on soil protozoa. Biol. Fertil. Soils 40 230–236. 10.1007/s00374-004-0774-y - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

