Anti-FIM and Anti-FHA Antibodies Inhibit Bordetella pertussis Growth and Reduce Epithelial Cell Inflammation Through Bacterial Aggregation
- PMID: 33384692
- PMCID: PMC7770163
- DOI: 10.3389/fimmu.2020.605273
Anti-FIM and Anti-FHA Antibodies Inhibit Bordetella pertussis Growth and Reduce Epithelial Cell Inflammation Through Bacterial Aggregation
Abstract
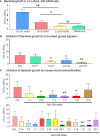
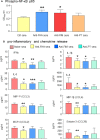
The pertussis vaccination is highly recommended for infants, children, and pregnant women. Despite a high coverage of vaccination, pertussis continues to be of public health concern as a re-emerging infectious disease. The mechanism by which vaccine-elicited anti-pertussis antibodies mediate direct bactericidal effects is poorly understood. In this study, we showed that the interaction of B. pertussis with A549 epithelial cells induce release of biological factors which enhance bacteria growth. Complement-depleted antisera from vaccine-immunized guinea pigs or monoclonal antibodies targeting FHA and FIM mediate bacteria aggregation and elicit bactericidal effects. Our in vitro results indicated that aggregation of bacteria through anti-FIM and anti-FHA specific antibodies is one of the major biological mechanisms to clear bacterial infections and restore epithelial cell survival in vitro. Our data also indicates that the anti-pertussis antibodies reduce secretion of proinflammatory chemokines and cytokines by preventing interaction of B. pertussis with host cells. The results of this study not only demonstrate mechanism of action of anti-FIM and anti-FHA antibodies, but also opens translational applications for potential therapeutic approaches or development of analytical assays such as in vitro potency assays.
Keywords: aggregation; anti-pertussis antibodies; bacterial growth inhibition; cytokines; epithelial inflammation.
Copyright © 2020 Yougbare, McTague, He, Choy, Su, Gajewska and Azizi.
Conflict of interest statement
All authors are employees of Sanofi Pasteur.
Figures


References
-
- Koepke R, Eickhoff JC, Ayele RA, Petit AB, Schauer SL, Hopfensperger DJ, et al. Estimating the effectiveness of tetanus-diphtheria-acellular pertussis vaccine (Tdap) for preventing pertussis: evidence of rapidly waning immunity and difference in effectiveness by Tdap brand. J Infect Dis (2014) 210:942–53. 10.1093/infdis/jiu322 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

