Oculomotor Responses to Dynamic Stimuli in a 44-Channel Suprachoroidal Retinal Prosthesis
- PMID: 33384885
- PMCID: PMC7757638
- DOI: 10.1167/tvst.9.13.31
Oculomotor Responses to Dynamic Stimuli in a 44-Channel Suprachoroidal Retinal Prosthesis
Abstract
Purpose: To investigate oculomotor behavior in response to dynamic stimuli in retinal implant recipients.
Methods: Three suprachoroidal retinal implant recipients performed a four-alternative forced-choice motion discrimination task over six sessions longitudinally. Stimuli were a single white bar ("moving bar") or a series of white bars ("moving grating") sweeping left, right, up, or down across a 42″ monitor. Performance was compared with normal video processing and scrambled video processing (randomized image-to-electrode mapping to disrupt spatiotemporal structure). Eye and head movement was monitored throughout the task.
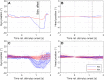
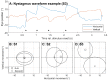
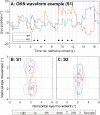
Results: Two subjects had diminished performance with scrambling, suggesting retinotopic discrimination was used in the normal condition and made smooth pursuit eye movements congruent to the moving bar stimulus direction. These two subjects also made stimulus-related eye movements resembling optokinetic reflex (OKR) for moving grating stimuli, but the movement was incongruent with stimulus direction. The third subject was less adept at the task, appeared primarily reliant on head position cues (head movements were congruent to stimulus direction), and did not exhibit retinotopic discrimination and associated eye movements.
Conclusions: Our observation of smooth pursuit indicates residual functionality of cortical direction-selective circuits and implies a more naturalistic perception of motion than expected. A distorted OKR implies improper functionality of retinal direction-selective circuits, possibly due to retinal remodeling or the non-selective nature of the electrical stimulation.
Translational relevance: Retinal implant users can make naturalistic eye movements in response to moving stimuli, highlighting the potential for eye tracker feedback to improve perceptual localization and image stabilization in camera-based visual prostheses.
Keywords: eye movements; head movements; motion perception; retinal prosthesis; visual prosthesis.
Copyright 2020 The Authors.
Conflict of interest statement
Disclosure: S.A. Titchener, Bionic Vision Technologies Pty Ltd (F); J. Kvansakul, Bionic Vision Technologies Pty Ltd (F); M.N. Shivdasani (P); J.B. Fallon, None; D.A.X. Nayagam, Bionic Vision Technologies Pty Ltd (F, P); S.B. Epp, Bionic Vision Technologies Pty Ltd (F); C.E. Williams, Bionic Vision Technologies Pty Ltd (F, P); N. Barnes, Bionic Vision Technologies Pty Ltd (F); W.G. Kentler, None; M. Kolic, Bionic Vision Technologies Pty Ltd (F); E.K. Baglin, Bionic Vision Technologies Pty Ltd (F); L.N. Ayton, None; C.J. Abbott, Bionic Vision Technologies Pty Ltd (F); C.D. Luu, Bionic Vision Technologies Pty Ltd (F, P); P.J. Allen, Bionic Vision Technologies Pty Ltd (F, P); M.A. Petoe, Bionic Vision Technologies Pty Ltd (F, P)
Figures



References
-
- Luo YH-L, da Cruz L. The Argus II Retinal Prosthesis System. Prog Retin Eye Res. 2016; 50: 89–107. - PubMed
-
- Muqit MMK, Velikay-Parel M, Weber M, et al.. Six-month safety and efficacy of the Intelligent Retinal Implant System II device in retinitis pigmentosa. Ophthalmology. 2019; 126(4): 637–639. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

