Antenatal Corticosteroid Therapy Attenuates Angiogenesis Through Inhibiting Osteoclastogenesis in Young Mice
- PMID: 33384997
- PMCID: PMC7769874
- DOI: 10.3389/fcell.2020.601188
Antenatal Corticosteroid Therapy Attenuates Angiogenesis Through Inhibiting Osteoclastogenesis in Young Mice
Abstract
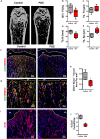
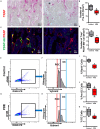
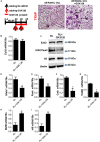
Antenatal corticosteroid therapy (ACT) has been shown to reduce morbidity and mortality rates in preterm delivery, but the fetus is more likely to face the risk of low bone mineralization and low fetal linear growth. However, the mechanism of ACT inducing low bone mineralization remains largely unknown. Pre-osteoclasts, which play an important role in angiogenesis and osteogenesis, are specifically regulating type H vessels (CD31hiEmcnhi) and vessel formation by secreting platelet-derived growth factor-BB (PDGF-BB). We find that the number of pre-osteoclasts and POC-secreted PDGF-BB is dramatically decreased in ACT mice, contributing to the reduction in type H vessels and bone mineralization during the mouse offspring. Quantitative analyses of micro-computed tomography show that the ACT mice have a significant reduction in the mass of trabecular bone relative to the control group. Mononuclear pre-osteoclasts in trabecular bone decreased in ACT mice, which leads to the amount of PDGF-BB reduced and attenuates type H vessel formation. After sorting the Rank+ osteoclast precursors using flow cytometry, we show that the enhancer of zeste homolog 2 (Ezh2) expression is decreased in Rank+ osteoclast precursors in ACT mice. Consistent with the flow data, by using small molecule Ezh2 inhibitor GSK126, we prove that Ezh2 is required for osteoclast differentiation. Downregulating the expression of Ezh2 in osteoclast precursors would reduce PDGF-BB production. Conditioned medium from osteoclast precursor cultures treated with GSK126 inhibited endothelial tube formation, whereas conditioned medium from vehicle group stimulated endothelial tube formation. These results indicate Ezh2 expression of osteoclast precursors is suppressed after ACT, which reduced the pre-osteoclast number and PDGF-BB secretion, thus inhibiting type H vessel formation and ACT-associated low bone mineralization.
Keywords: ACT; EZH2; angiogenesis; osteoclastogenesis; pre-osteoclasts.
Copyright © 2020 Chai, Su, Hong, Zhu, Cheng, Wang, Zhang and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
GIT1 is critical for formation of the CD31hiEmcnhi vessel subtype in coupling osteogenesis with angiogenesis via modulating preosteoclasts secretion of PDGF-BB.Bone. 2019 May;122:218-230. doi: 10.1016/j.bone.2019.03.006. Epub 2019 Mar 7. Bone. 2019. PMID: 30853660
-
Glucocorticoids Disrupt Skeletal Angiogenesis Through Transrepression of NF-κB-Mediated Preosteoclast Pdgfb Transcription in Young Mice.J Bone Miner Res. 2020 Jun;35(6):1188-1202. doi: 10.1002/jbmr.3987. Epub 2020 Mar 11. J Bone Miner Res. 2020. PMID: 32078184 Free PMC article.
-
PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis.Nat Med. 2014 Nov;20(11):1270-8. doi: 10.1038/nm.3668. Epub 2014 Oct 5. Nat Med. 2014. PMID: 25282358 Free PMC article.
-
The molecular basis of osteoclast differentiation and activation.Novartis Found Symp. 2001;232:235-47; discussion 247-50. doi: 10.1002/0470846658.ch16. Novartis Found Symp. 2001. PMID: 11277084 Review.
-
Type H vessels: functions in bone development and diseases.Front Cell Dev Biol. 2023 Nov 16;11:1236545. doi: 10.3389/fcell.2023.1236545. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38033859 Free PMC article. Review.
Cited by
-
Targeting type H vessels in bone-related diseases.J Cell Mol Med. 2024 Feb;28(4):e18123. doi: 10.1111/jcmm.18123. J Cell Mol Med. 2024. PMID: 38353470 Free PMC article. Review.
-
Prenatal dexamethasone exposure reduces osteoprogenitor proliferation in mice via histone modifications at the Mkp-1 gene locus.Commun Biol. 2024 Nov 28;7(1):1589. doi: 10.1038/s42003-024-07288-x. Commun Biol. 2024. PMID: 39609620 Free PMC article.
-
Glucocorticoid induced bone disorders in children: Research progress in treatment mechanisms.Front Endocrinol (Lausanne). 2023 Apr 4;14:1119427. doi: 10.3389/fendo.2023.1119427. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37082116 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

