In vitro amplification of pathogenic tau conserves disease-specific bioactive characteristics
- PMID: 33385254
- PMCID: PMC7847465
- DOI: 10.1007/s00401-020-02253-4
In vitro amplification of pathogenic tau conserves disease-specific bioactive characteristics
Abstract
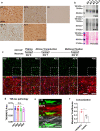
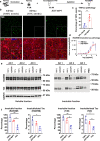
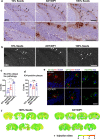
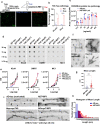
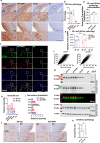
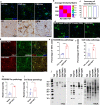
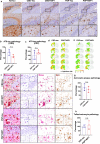
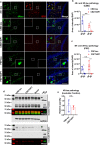
The microtubule-associated protein tau (tau) forms hyperphosphorylated aggregates in the brains of tauopathy patients that can be pathologically and biochemically defined as distinct tau strains. Recent studies show that these tau strains exhibit strain-specific biological activities, also referred to as pathogenicities, in the tau spreading models. Currently, the specific pathogenicity of human-derived tau strains cannot be fully recapitulated by synthetic tau preformed fibrils (pffs), which are generated from recombinant tau protein. Reproducing disease-relevant tau pathology in cell and animal models necessitates the use of human brain-derived tau seeds. However, the availability of human-derived tau is extremely limited. Generation of tau variants that can mimic the pathogenicity of human-derived tau seeds would significantly extend the scale of experimental design within the field of tauopathy research. Previous studies have demonstrated that in vitro seeding reactions can amplify the beta-sheet structure of tau protein from a minute quantity of human-derived tau. However, whether the strain-specific pathogenicities of the original, human-derived tau seeds are conserved in the amplified tau strains has yet to be experimentally validated. Here, we used biochemically enriched brain-derived tau seeds from Alzheimer's disease (AD), corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP) patient brains with a modified seeding protocol to template the recruitment of recombinant 2N4R (T40) tau in vitro. We quantitatively interrogated efficacy of the amplification reactions and the pathogenic fidelity of the amplified material to the original tau seeds using recently developed sporadic tau spreading models. Our data suggest that different tau strains can be faithfully amplified in vitro from tau isolated from different tauopathy brains and that the amplified tau variants retain their strain-dependent pathogenic characteristics.
Keywords: in vitro seeding; tau spreading; tau strains; tauopathy.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

