Preclinical techniques to investigate exercise training in vascular pathophysiology
- PMID: 33385323
- PMCID: PMC8260379
- DOI: 10.1152/ajpheart.00719.2020
Preclinical techniques to investigate exercise training in vascular pathophysiology
Abstract
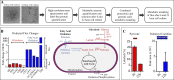
Atherosclerosis is a dynamic process starting with endothelial dysfunction and inflammation and eventually leading to life-threatening arterial plaques. Exercise generally improves endothelial function in a dose-dependent manner by altering hemodynamics, specifically by increased arterial pressure, pulsatility, and shear stress. However, athletes who regularly participate in high-intensity training can develop arterial plaques, suggesting alternative mechanisms through which excessive exercise promotes vascular disease. Understanding the mechanisms that drive atherosclerosis in sedentary versus exercise states may lead to novel rehabilitative methods aimed at improving exercise compliance and physical activity. Preclinical tools, including in vitro cell assays, in vivo animal models, and in silico computational methods, broaden our capabilities to study the mechanisms through which exercise impacts atherogenesis, from molecular maladaptation to vascular remodeling. Here, we describe how preclinical research tools have and can be used to study exercise effects on atherosclerosis. We then propose how advanced bioengineering techniques can be used to address gaps in our current understanding of vascular pathophysiology, including integrating in vitro, in vivo, and in silico studies across multiple tissue systems and size scales. Improving our understanding of the antiatherogenic exercise effects will enable engaging, targeted, and individualized exercise recommendations to promote cardiovascular health rather than treating cardiovascular disease that results from a sedentary lifestyle.
Keywords: atherosclerosis; bionengineering; cardiovascular; exercise; preclinical.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

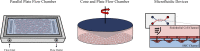
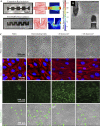
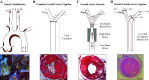
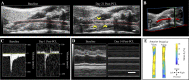

Similar articles
-
Exercise interventions and peripheral arterial function: implications for cardio-metabolic disease.Prog Cardiovasc Dis. 2015 Mar-Apr;57(5):521-34. doi: 10.1016/j.pcad.2014.12.005. Epub 2014 Dec 18. Prog Cardiovasc Dis. 2015. PMID: 25529367 Review.
-
Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression.Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):790-800. doi: 10.1161/ATVBAHA.113.303116. Epub 2014 Feb 13. Arterioscler Thromb Vasc Biol. 2014. PMID: 24526691
-
Arterial ageing: from endothelial dysfunction to vascular calcification.J Intern Med. 2017 May;281(5):471-482. doi: 10.1111/joim.12605. Epub 2017 Mar 27. J Intern Med. 2017. PMID: 28345303 Review.
-
Exercise training and vascular cell phenotype in a swine model of familial hypercholesterolaemia: conduit arteries and veins.Exp Physiol. 2014 Feb;99(2):454-65. doi: 10.1113/expphysiol.2013.075838. Epub 2013 Nov 8. Exp Physiol. 2014. PMID: 24213857 Free PMC article.
-
Endothelial loss and repair in the vascular complications of diabetes: pathogenetic mechanisms and therapeutic implications.Circ J. 2013;77(4):849-56. doi: 10.1253/circj.cj-13-0236. Epub 2013 Mar 16. Circ J. 2013. PMID: 23503045 Review.
Cited by
-
The impact of acute and chronic aerobic and resistance exercise on stem cell mobilization: A review of effects in healthy and diseased individuals across different age groups.Regen Ther. 2024 May 7;27:464-481. doi: 10.1016/j.reth.2024.04.013. eCollection 2024 Dec. Regen Ther. 2024. PMID: 38745840 Free PMC article.
-
Vascular calcification: a left-handed compliment for aging.Am J Physiol Heart Circ Physiol. 2021 Aug 1;321(2):H422-H423. doi: 10.1152/ajpheart.00300.2021. Epub 2021 Jul 23. Am J Physiol Heart Circ Physiol. 2021. PMID: 34296967 Free PMC article. No abstract available.
-
Restoration of normal blood flow in atherosclerotic arteries promotes plaque stabilization.iScience. 2023 Apr 27;26(6):106760. doi: 10.1016/j.isci.2023.106760. eCollection 2023 Jun 16. iScience. 2023. PMID: 37235059 Free PMC article.
-
Association between Urinary Phthalate Metabolites and Markers of Endothelial Dysfunction in Adolescents and Young Adults.Toxics. 2021 Feb 6;9(2):33. doi: 10.3390/toxics9020033. Toxics. 2021. PMID: 33562063 Free PMC article.
-
Microfluidic investigation for shear-stress-mediated repair of dysglycemia-induced endothelial cell damage.Mechanobiol Med. 2024 Apr 29;2(3):100069. doi: 10.1016/j.mbm.2024.100069. eCollection 2024 Sep. Mechanobiol Med. 2024. PMID: 40395495 Free PMC article. Review.
References
-
- Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Xuemei S, Wisløff U, Stroke Council. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 134: e653–e699, 2016. - PubMed
-
- World Health Organization. Global Recommendations on Physical Activity for Health. Geneva, Switzerland: World Health Organization, 2010. - PubMed
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, On behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 141: e139–e596, 2020. doi:10.1161/CIR.0000000000000757. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

