Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies
- PMID: 33385336
- PMCID: PMC7878403
- DOI: 10.1016/j.chom.2020.12.002
Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies
Abstract
Candida auris is an emerging multi-drug-resistant human fungal pathogen. C. auris skin colonization results in environmental shedding, which underlies hospital transmissions, and predisposes patients to subsequent infections. We developed a murine skin topical exposure model for C. auris to dissect risk factors for colonization and to test interventions that might protect patients. We demonstrate that C. auris establishes long-term residence within the skin tissue compartment, which would elude clinical surveillance. The four clades of C. auris, with geographically distinct origins, differ in their abilities to colonize murine skin, mirroring epidemiologic findings. The IL-17 receptor signaling and specific arms of immunity protect mice from long-term C. auris skin colonization. We further determine that commonly used chlorhexidine antiseptic serves as a protective and decolonizing agent against C. auris. This translational model facilitates an integrated approach to develop strategies to combat the unfolding global outbreaks of C. auris and other skin-associated microbial pathogens.
Keywords: Candida auris; adaptive and innate immunity; epidemiology; fungal pathogen; skin colonization; translational mouse model.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
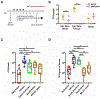
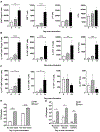


Comment in
-
Exploring Candida auris in its habitat.Cell Host Microbe. 2021 Feb 10;29(2):150-151. doi: 10.1016/j.chom.2021.01.010. Cell Host Microbe. 2021. PMID: 33571440 Free PMC article.
References
-
- Cassir N, Papazian L, Fournier PE, Raoult D, and La Scola B (2015). Insights into bacterial colonization of intensive care patients' skin: the effect of chlorhexidine daily bathing. Eur J Clin Microbiol. 34(5), 999–1004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

